Intrinsic functional defects of type 2 innate lymphoid cells impair innate allergic inflammation in promyelocytic leukemia zinc finger (PLZF)-deficient mice
- PMID: 26602165
- PMCID: PMC4747811
- DOI: 10.1016/j.jaci.2015.07.050
Intrinsic functional defects of type 2 innate lymphoid cells impair innate allergic inflammation in promyelocytic leukemia zinc finger (PLZF)-deficient mice
Abstract
Background: The transcription factor promyelocytic leukemia zinc finger (PLZF) is transiently expressed during development of type 2 innate lymphoid cells (ILC2s) but is not present at the mature stage. We hypothesized that PLZF-deficient ILC2s have functional defects in the innate allergic response and represent a tool for studying innate immunity in a mouse with a functional adaptive immune response.
Objective: We determined the consequences of PLZF deficiency on ILC2 function in response to innate and adaptive immune stimuli by using PLZF(-/-) mice and mixed wild-type:PLZF(-/-) bone marrow chimeras.
Methods: PLZF(-/-) mice, wild-type littermates, or mixed bone marrow chimeras were treated with the protease allergen papain or the cytokines IL-25 and IL-33 or infected with the helminth Nippostrongylus brasiliensis to induce innate type 2 allergic responses. Mice were sensitized with intraperitoneal ovalbumin-alum, followed by intranasal challenge with ovalbumin alone, to induce adaptive TH2 responses. Lungs were analyzed for immune cell subsets, and alveolar lavage fluid was analyzed for ILC2-derived cytokines. In addition, ILC2s were stimulated ex vivo for their capacity to release type 2 cytokines.
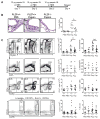
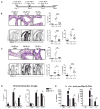
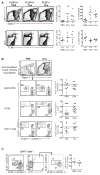
Results: PLZF-deficient lung ILC2s exhibit a cell-intrinsic defect in the secretion of IL-5 and IL-13 in response to innate stimuli, resulting in defective recruitment of eosinophils and goblet cell hyperplasia. In contrast, the adaptive allergic inflammatory response to ovalbumin and alum was unimpaired.
Conclusions: PLZF expression at the innate lymphoid cell precursor stage has a long-range effect on the functional properties of mature ILC2s and highlights the importance of these cells for innate allergic responses in otherwise immunocompetent mice.
Keywords: Allergic mechanisms; innate lymphoid cells; mouse models.
Copyright © 2015 American Academy of Allergy, Asthma & Immunology. All rights reserved.
Conflict of interest statement
Conflicts of interest: none
Figures


References
-
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nature reviews Immunology. 2013;13(2):101–17. - PubMed
-
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. - PubMed
-
- Fuchs A, Colonna M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr Opin Gastroenterol. 2013;29(6):581–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL118092/HL/NHLBI NIH HHS/United States
- T32 GM007281/GM/NIGMS NIH HHS/United States
- R01 AI108643/AI/NIAID NIH HHS/United States
- R01AI108643/AI/NIAID NIH HHS/United States
- R01AI038339/AI/NIAID NIH HHS/United States
- P30DK42086/DK/NIDDK NIH HHS/United States
- P30 DK042086/DK/NIDDK NIH HHS/United States
- R01 HL118092/HL/NHLBI NIH HHS/United States
- T32 HL007605/HL/NHLBI NIH HHS/United States
- K12 HL119995/HL/NHLBI NIH HHS/United States
- AI108643/AI/NIAID NIH HHS/United States
- R01 AI038339/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

