Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1
- PMID: 26602187
- PMCID: PMC4752817
- DOI: 10.1016/j.str.2015.09.010
Structure of the Complex of Human Programmed Death 1, PD-1, and Its Ligand PD-L1
Abstract
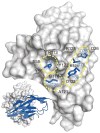
Targeting the PD-1/PD-L1 immunologic checkpoint with monoclonal antibodies has recently provided breakthrough progress in the treatment of melanoma, non-small cell lung cancer, and other types of cancer. Small-molecule drugs interfering with this pathway are highly awaited, but their development is hindered by insufficient structural information. This study reveals the molecular details of the human PD-1/PD-L1 interaction based on an X-ray structure of the complex. First, it is shown that the ligand binding to human PD-1 is associated with significant plasticity within the receptor. Second, a detailed molecular map of the interaction surface is provided, allowing definition of the regions within both interacting partners that may likely be targeted by small molecules.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
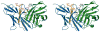
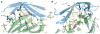

References
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
-
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

