Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States
- PMID: 26602547
- PMCID: PMC5040950
- DOI: 10.1016/j.cllc.2015.07.005
Sublobar Resection for Clinical Stage IA Non-small-cell Lung Cancer in the United States
Abstract
Background: This study evaluated the use of lobectomy and sublobar resection for clinical stage IA non-small-cell lung cancer (NSCLC) in the National Cancer Data Base (NCDB).
Methods: The NCDB from 2003 to 2011 was analyzed to determine factors associated with the use of a sublobar resection versus a lobectomy for the treatment of clinical stage IA NSCLC. Overall survival was assessed using the Kaplan-Meier method and Cox proportional hazard modeling.
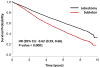
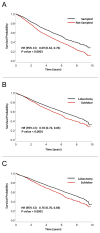
Results: Among 39,403 patients included for analysis, 29,736 (75.5%) received a lobectomy and 9667 (24.5%) received a sublobar resection: 84.7% wedge resection (n = 8192) and 15.3% segmental resection (n = 1475). Lymph node evaluation was not performed in 2788 (28.8%) of sublobar resection patients, and 7298 (75.5%) of sublobar resections were for tumors ≤ 2 cm. After multivariable logistic regression, older age, higher Charlson-Deyo comorbidity scores, smaller tumor size, and treatment at lower-volume institutions were associated with sublobar resection (all P < .001). Overall, lobectomy was associated with significantly improved 5-year survival compared to sublobar resection (66.2% vs. 51.2%; P < .001, adjusted hazard ratio 0.66; P < .001). However among sublobar resection patients, nodal sampling was associated with significantly better 5-year survival (58.2% vs. 46.4%; P < .001).
Conclusion: Despite adjustment for patient and tumor related characteristics, a sublobar resection is associated with significantly reduced long-term survival compared to a formal surgical lobectomy among patients with NSCLC, even for stage 1A tumors. For patients who cannot tolerate lobectomy and who are treated with sublobar resection, lymph node evaluation is essential to help guide further treatment.
Keywords: Lobectomy; Lung cancer; NSCLC; Stage IA; Sublobar resection.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
One of the authors (T.A.D.) serves as a consultant for Scanlan International, Inc.
Figures
References
-
- Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer, version 2.2013. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:645–53. quiz 53. - PubMed
-
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of thoracic surgery. 1995;60:615–22. discussion 22–3. - PubMed
-
- Altorki NK, Yip R, Hanaoka T, Bauer T, Aye R, Kohman L, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. The Journal of thoracic and cardiovascular surgery. 2014;147:754–62. Discussion 62–4. - PubMed
-
- El-Sherif A, Gooding WE, Santos R, Pettiford B, Ferson PF, Fernando HC, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. The Annals of thoracic surgery. 2006;82:408–15. discussion 15–6. - PubMed
-
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J. 2009;33(2):426–35. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

