In Vivo and In Silico Investigation Into Mechanisms of Frequency Dependence of Repolarization Alternans in Human Ventricular Cardiomyocytes
- PMID: 26602864
- PMCID: PMC4719495
- DOI: 10.1161/CIRCRESAHA.115.307836
In Vivo and In Silico Investigation Into Mechanisms of Frequency Dependence of Repolarization Alternans in Human Ventricular Cardiomyocytes
Abstract
Rationale: Repolarization alternans (RA) are associated with arrhythmogenesis. Animal studies have revealed potential mechanisms, but human-focused studies are needed. RA generation and frequency dependence may be determined by cell-to-cell variability in protein expression, which is regulated by genetic and external factors.
Objective: To characterize in vivo RA in human and to investigate in silico using human models, the ionic mechanisms underlying the frequency-dependent differences in RA behavior identified in vivo.
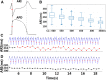
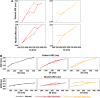
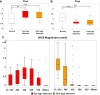
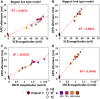
Methods and results: In vivo electrograms were acquired at 240 sites covering the epicardium of 41 patients at 6 cycle lengths (600-350 ms). In silico investigations were conducted using a population of biophysically detailed human models incorporating variability in protein expression and calibrated using in vivo recordings. Both in silico and in vivo, 2 types of RA were identified, with Fork- and Eye-type restitution curves, based on RA persistence or disappearance, respectively, at fast pacing rates. In silico simulations show that RA are strongly correlated with fluctuations in sarcoplasmic reticulum calcium, because of strong release and weak reuptake. Large L-type calcium current conductance is responsible for RA disappearance at fast frequencies in Eye-type (30% larger in Eye-type versus Fork-type; P<0.01), because of sarcoplasmic reticulum Ca(2+) ATPase pump potentiation caused by frequency-induced increase in intracellular calcium. Large Na(+)/Ca(2+) exchanger current is the main driver in translating Ca(2+) fluctuations into RA.
Conclusions: In human in vivo and in silico, 2 types of RA are identified, with RA persistence/disappearance as frequency increases. In silico, L-type calcium current and Na(+)/Ca(2+) exchanger current determine RA human cell-to-cell differences through intracellular and sarcoplasmic reticulum calcium regulation.
Keywords: calcium; calibration; electrophysiology; pericardium; sarcoplasmic reticulum.
© 2015 The Authors.
Figures



Comment in
-
From Single Myocyte to Whole Heart: The Intricate Dance of Electrophysiology and Modeling.Circ Res. 2016 Jan 22;118(2):184-6. doi: 10.1161/CIRCRESAHA.115.308067. Circ Res. 2016. PMID: 26838308 Free PMC article. No abstract available.
References
-
- Armoundas AA, Tomaselli GF, Esperer HD. Pathophysiological basis and clinical application of T-wave alternans. J Am Coll Cardiol. 2002;40:207–217. - PubMed
-
- Armoundas AA, Hohnloser SH, Ikeda T, Cohen RJ. Can microvolt T-wave alternans testing reduce unnecessary defibrillator implantation? Nat Clin Pract Cardiovasc Med. 2005;2:522–528. doi: 10.1038/ncpcardio0323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

