Use of biochemical tests of placental function for improving pregnancy outcome
- PMID: 26602956
- PMCID: PMC8860184
- DOI: 10.1002/14651858.CD011202.pub2
Use of biochemical tests of placental function for improving pregnancy outcome
Abstract
Background: The placenta has an essential role in determining the outcome of pregnancy. Consequently, biochemical measurement of placentally-derived factors has been suggested as a means to improve fetal and maternal outcome of pregnancy.
Objectives: To assess whether clinicians' knowledge of the results of biochemical tests of placental function is associated with improvement in fetal or maternal outcome of pregnancy.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 July 2015) and reference lists of retrieved studies.
Selection criteria: Randomised, cluster-randomised or quasi-randomised controlled trials assessing the merits of the use of biochemical tests of placental function to improve pregnancy outcome.Studies were eligible if they compared women who had placental function tests and the results were available to their clinicians with women who either did not have the tests, or the tests were done but the results were not available to the clinicians. The placental function tests were any biochemical test of placental function carried out using the woman's maternal biofluid, either alone or in combination with other placental function test/s.
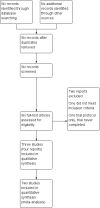
Data collection and analysis: Two review authors independently assessed trials for inclusion, extracted data and assessed trial quality. Authors of published trials were contacted for further information.
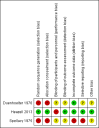
Main results: Three trials were included, two quasi-randomised controlled trials and one randomised controlled trial. One trial was deemed to be at low risk of bias while the other two were at high risk of bias. Different biochemical analytes were measured - oestrogen was measured in one trial and the other two measured human placental lactogen (hPL). One trial did not contribute outcome data, therefore, the results of this review are based on two trials with 740 participants.There was no evidence of a difference in the incidence of death of a baby (risk ratio (RR) 0.88, 95% confidence interval (CI) 0.36 to 2.13, two trials, 740 participants (very low quality evidence)) or the frequency of a small-for-gestational-age infant (RR 0.44, 95% CI 0.16 to 1.19, one trial, 118 participants (low quality evidence)).In terms of this review's secondary outcomes, there was no evidence of a clear difference between women who had biochemical tests of placental function compared with standard antenatal care for the incidence of stillbirth (RR 0.56, 95% CI 0.16 to 1.88, two trials, 740 participants (very low quality evidence)) or neonatal death (RR 1.62, 95% CI 0.39 to 6.74, two trials, 740 participants, very low quality evidence)) although the directions of any potential effect were in opposing directions. There was no evidence of a difference between groups in elective delivery (RR 0.98, 95% CI 0.84 to 1.14, two trials, 740 participants (low quality evidence)), caesarean section (one trial, RR 0.48, 95% CI 0.15 to 1.52, one trial, 118 participants (low quality evidence)), change in anxiety score (mean difference -2.40, 95% CI -4.78 to -0.02, one trial, 118 participants), admissions to neonatal intensive care (RR 0.32, 95% CI 0.03 to 3.01, one trial, 118 participants), and preterm birth before 37 weeks' gestation (RR 2.90, 95% CI 0.12 to 69.81, one trial, 118 participants). One trial (118 participants) reported that there were no cases of serious neonatal morbidity. Maternal death was not reported.A number of this review's secondary outcomes relating to the baby were not reported in the included studies, namely: umbilical artery pH < 7.0, neonatal intensive care for more than seven days, very preterm birth (< 32 weeks' gestation), need for ventilation, organ failure, fetal abnormality, neurodevelopment in childhood (cerebral palsy, neurodevelopmental delay). Similarly, a number of this review's maternal secondary outcomes were not reported in the included studies (admission to intensive care, high dependency unit admission, hospital admission for > seven days, pre-eclampsia, eclampsia, and women's perception of care).
Authors' conclusions: There is insufficient evidence to support the use of biochemical tests of placental function to reduce perinatal mortality or increase identification of small-for-gestational-age infants. However, we were only able to include data from two studies that measured oestrogens and hPL. The quality of the evidence was low or very low.Two of the trials were performed in the 1970s on women with a variety of antenatal complications and this evidence cannot be generalised to women at low-risk of complications or groups of women with specific pregnancy complications (e.g. fetal growth restriction). Furthermore, outcomes described in the 1970s may not reflect what would be expected at present. For example, neonatal mortality rates have fallen substantially, such that an infant delivered at 28 weeks would have a greater chance of survival were those studies repeated; this may affect the primary outcome of the meta-analysis.With data from just two studies (740 women), this review is underpowered to detect a difference in the incidence of death of a baby or the frequency of a small-for-gestational-age infant as these have a background incidence of approximately 0.75% and 10% of pregnancies respectively. Similarly, this review is underpowered to detect differences between serious and/or rare adverse events such as severe neonatal morbidity. Two of the three included studies were quasi-randomised, with significant risk of bias from group allocation. Additionally, there may be performance bias as in one of the two studies contributing data, participants receiving standard care did not have venepuncture, so clinicians treating participants could identify which arm of the study they were in. Future studies should consider more robust randomisation methods and concealment of group allocation and should be adequately powered to detect differences in rare adverse events.The studies identified in this review examined two different analytes: oestrogens and hPL. There are many other placental products that could be employed as surrogates of placental function, including: placental growth factor (PlGF), human chorionic gonadotrophin (hCG), plasma protein A (PAPP-A), placental protein 13 (PP-13), pregnancy-specific glycoproteins and progesterone metabolites and further studies should be encouraged to investigate these other placental products. Future randomised controlled trials should test analytes identified as having the best predictive reliability for placental dysfunction leading to small-for-gestational-age infants and perinatal mortality.
Conflict of interest statement
Jim Thornton and Melissa Whitworth: none known.
Lelia Duley has been awarded an NIHR applied research grant for a programme of work on care at very preterm birth. She is also a collaborator on Alexander Heazell's NIHR Clinician Scientist award which includes funding for a randomised trial (which will be conducted by the Nottingham Clinical Trials Unit) relevant to this review.
Alexander Heazell has received research grants from Alere (UK) and Action Medical Research to investigate placental factors in maternal serum in women with reduced fetal movements. Alexander Heazell holds a Clinician Scientist Award from NIHR and this award includes funding for a randomised trial (which will be conducted by the Nottingham Clinical Trials Unit) relevant to this review. Alexander Heazell was the trialist for one of the included studies (Heazell 2013), he was not directly responsible for decisions involving the inclusion, assessment of quality or data extraction for this study. These tasks were carried out by members of the review team not directly involved with this study.
Figures










Update of
- doi: 10.1002/14651858.CD011202
Similar articles
-
Antenatal dietary supplementation with myo-inositol in women during pregnancy for preventing gestational diabetes.Cochrane Database Syst Rev. 2015 Dec 17;2015(12):CD011507. doi: 10.1002/14651858.CD011507.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2023 Feb 15;2:CD011507. doi: 10.1002/14651858.CD011507.pub3. PMID: 26678256 Free PMC article. Updated.
-
Planned early delivery versus expectant management of the term suspected compromised baby for improving outcomes.Cochrane Database Syst Rev. 2015 Nov 24;2015(11):CD009433. doi: 10.1002/14651858.CD009433.pub2. Cochrane Database Syst Rev. 2015. PMID: 26599471 Free PMC article.
-
Sealing procedures for preterm prelabour rupture of membranes.Cochrane Database Syst Rev. 2016 Jul 7;7(7):CD010218. doi: 10.1002/14651858.CD010218.pub2. Cochrane Database Syst Rev. 2016. PMID: 27384151 Free PMC article.
-
Immediate versus deferred delivery of the preterm baby with suspected fetal compromise for improving outcomes.Cochrane Database Syst Rev. 2016 Jul 12;7(7):CD008968. doi: 10.1002/14651858.CD008968.pub3. Cochrane Database Syst Rev. 2016. PMID: 27404120 Free PMC article.
-
Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. Cochrane Database Syst Rev. 2015. PMID: 26671418 Free PMC article.
Cited by
-
Interventions during pregnancy to prevent preterm birth: an overview of Cochrane systematic reviews.Cochrane Database Syst Rev. 2018 Nov 14;11(11):CD012505. doi: 10.1002/14651858.CD012505.pub2. Cochrane Database Syst Rev. 2018. PMID: 30480756 Free PMC article.
-
The PLANES study: a protocol for a randomised controlled feasibility study of the placental growth factor (PlGF) blood test-informed care versus standard care alone for women with a small for gestational age fetus at or after 32 + 0 weeks' gestation.Pilot Feasibility Stud. 2020 Nov 19;6(1):179. doi: 10.1186/s40814-020-00722-x. Pilot Feasibility Stud. 2020. PMID: 33292754 Free PMC article.
-
Exploring in vivo placental microstructure in healthy and growth-restricted pregnancies through diffusion-weighted magnetic resonance imaging.Placenta. 2020 Apr;93:113-118. doi: 10.1016/j.placenta.2020.03.004. Epub 2020 Mar 6. Placenta. 2020. PMID: 32250735 Free PMC article.
-
Antenatal interventions for preventing stillbirth, fetal loss and perinatal death: an overview of Cochrane systematic reviews.Cochrane Database Syst Rev. 2020 Dec 18;12(12):CD009599. doi: 10.1002/14651858.CD009599.pub2. Cochrane Database Syst Rev. 2020. PMID: 33336827 Free PMC article.
-
Ultrasound based radiomics model for assessment of placental function in pregnancies with preeclampsia.Sci Rep. 2024 Sep 10;14(1):21123. doi: 10.1038/s41598-024-72046-2. Sci Rep. 2024. PMID: 39256496 Free PMC article.
References
References to studies included in this review
Duenhoelter 1976 {published data only}
-
- Duenhoelter JH, Whalley PJ, MacDonald PC. An analysis of the utility of plasma immunoreactive estrogen measurements in determining delivery time of gravidas with a fetus considered at high risk. American Journal of Obstetrics and Gynecology 1976;125:889‐98. - PubMed
Heazell 2013 {published data only}
-
- Bernatavicius G, Roberts S, Garrod A, Whitworth MK, Johnstone ED, Gillham JC, et al. A feasibility study for a randomised controlled trial of management of reduced fetal movements after 36 weeks gestation. Archives of Disease in Childhood: Fetal and Neonatal Edition 2013;98(Suppl 1):A91. - PMC - PubMed
Spellacy 1975 {published data only}
-
- Spellacy WN, Buhi WC, Birk SA. The effectiveness of human placental lactogen measurements as an adjunct in decreasing perinatal deaths. Results of a retrospective and randomized controlled prospective study. American Journal of Obstetrics and Gynecology 1975;121:835‐44. - PubMed
References to studies excluded from this review
Grudzinskas 1990 {published data only}
-
- Grudzinskas JG. To assess the effects of biochemical placental function testing [trial abandoned]. Personal communication with The Cochrane Pregnancy and Childbirth Group 1990.
Sharf 1984 {published data only}
-
- Sharf, M. Eibschitz I, Hakim M, Degani S, Rosner B. Is serum free estriol measurement essential in the management of hypertensive disorders of pregnancy?. European Journal of Obstetrics and Gynaecology and Reproductive Biology 1984;17:365‐75. - PubMed
Additional references
Alfirevic 2013
Alfirevic 2015
Benton 2012
-
- Benton SJ, Hu Y, Xie F, Kupfer K, Lee SW, Magee LA, et al. Can placental growth factor in maternal circulation identify fetuses with placental intrauterine growth restriction?. American Journal of Obstetrics and Gynecology 2012;206(2):163.e1‐7. [PUBMED: 22055338] - PubMed
Bernatavicius 2013
-
- Bernatavicius G, Roberts S, Garrod A, Whitworth MK, Johnstone ED, Gillham JC, et al. A feasibility study for a randomised controlled trial of management of reduced fetal movements after 36 weeks gestation. Archives of Disease in Childhood: Fetal and Neonatal Edition 2013;98(Suppl 1):A91. - PMC - PubMed
Brosens 2011
Conde‐Agudelo 2013
-
- Conde‐Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2013;120(6):681‐94. [PUBMED: 23398929] - PubMed
Dugoff 2004
-
- Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First‐trimester maternal serum PAPP‐A and free‐beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population‐based screening study (the FASTER Trial). American Journal of Obstetrics and Gynecology 2004;191(4):1446‐51. [PUBMED: 15507981] - PubMed
Dutton 2012
Greene 1965
-
- Greene JW Jr, Duhring JL, Smith K. Placental function tests. A review of methods available for assessment of the fetoplacental complex. American Journal of Obstetrics and Gynecology 1965;92:1030‐58. [PUBMED: 14321104] - PubMed
Heazell 2015
-
- Heazell AE, Worton SA, Higgins LE, Ingram E, Johnstone ED, Jones RL, et al. Recognition of placental failure is key to saving babies' lives. Placenta 2015;36(Suppl 1):S20‐S28. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horgan 2011
-
- Horgan RP, Broadhurst DI, Walsh SK, Dunn WB, Brown M, Roberts CT, et al. Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy. Journal of Proteome Research 2011;10(8):3660‐73. [PUBMED: 21671558] - PubMed
Ness 2006
-
- Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. American Journal of Obstetrics and Gynecology 2006;195(1):40‐9. [PUBMED: 16813742] - PubMed
O'Sullivan 2009
-
- O'Sullivan O, Stephen G, Martindale E, Heazell AE. Predicting poor perinatal outcome in women who present with decreased fetal movements. Journal of Obstetrics and Gynaecology 2009;29(8):705‐10. - PubMed
Pinar 2014
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saffer 2013
-
- Saffer C, Olson G, Boggess KA, Beyerlein R, Eubank C, Sibai BM. Determination of placental growth factor (PlGF) levels in healthy pregnant women without signs or symptoms of preeclampsia. Hypertension in Pregnancy 2013;3(2):124‐32. - PubMed
Schneuer 2012
-
- Schneuer FJ, Nassar N, Khambalia AZ, Tasevski V, Guilbert C, Ashton AW, et al. First trimester screening of maternal placental protein 13 for predicting preeclampsia and small for gestational age: in‐house study and systematic review. Placenta 2012;33(9):735‐40. [PUBMED: 22748852] - PubMed
Smith 2007a
-
- Smith GC, Shah I, White IR, Pell JP, Crossley JA, Dobbie R. Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG: an international journal of obstetrics and gynaecology 2007;114(6):705‐14. [PUBMED: 17516962] - PubMed
Smith 2007b
-
- Smith GC, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstetrics and Gynecology 2007;109(6):1316‐24. [PUBMED: 17540803] - PubMed
References to other published versions of this review
Heazell 2014
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

