Artesunate-mefloquine versus chloroquine for treatment of uncomplicated Plasmodium knowlesi malaria in Malaysia (ACT KNOW): an open-label, randomised controlled trial
- PMID: 26603174
- PMCID: PMC4753673
- DOI: 10.1016/S1473-3099(15)00415-6
Artesunate-mefloquine versus chloroquine for treatment of uncomplicated Plasmodium knowlesi malaria in Malaysia (ACT KNOW): an open-label, randomised controlled trial
Abstract
Background: The zoonotic parasite Plasmodium knowlesi has become the most common cause of human malaria in Malaysia and is present throughout much of southeast Asia. No randomised controlled trials have been done to identify the optimum treatment for this emerging infection. We aimed to compare artesunate-mefloquine with chloroquine to define the optimum treatment for uncomplicated P knowlesi malaria in adults and children.
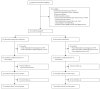
Methods: We did this open-label, randomised controlled trial at three district hospitals in Sabah, Malaysia. Patients aged 1 year or older with uncomplicated P knowlesi malaria were randomly assigned, via computer-generated block randomisation (block sizes of 20), to receive oral artesunate-mefloquine (target dose 12 mg/kg artesunate and 25 mg/kg mefloquine) or chloroquine (target dose 25 mg/kg). Research nursing staff were aware of group allocation, but allocation was concealed from the microscopists responsible for determination of the primary endpoint, and study participants were not aware of drug allocation. The primary endpoint was parasite clearance at 24 h. Analysis was by modified intention to treat. This study is registered with ClinicalTrials.gov, number NCT01708876.
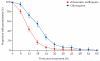
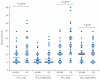
Findings: Between Oct 16, 2012, and Dec 13, 2014, we randomly assigned 252 patients to receive either artesunate-mefloquine (n=127) or chloroquine (n=125); 226 (90%) patients comprised the modified intention-to-treat population. 24 h after treatment, we recorded parasite clearance in 97 (84% [95% CI 76-91]) of 115 patients in the artesunate-mefloquine group versus 61 (55% [45-64]) of 111 patients in the chloroquine group (difference in proportion 29% [95% CI 18·0-40·8]; p<0·0001). Parasite clearance was faster in patients given artesunate-mefloquine than in those given chloroquine (18·0 h [range 6·0-48·0] vs 24·0 h [6·0-60·0]; p<0·0001), with faster clearance of ring stages in the artesunate-mefloquine group (mean time to 50% clearance of baseline parasites 8·6 h [95% CI 7·9-9·4] vs 13·8 h [12·1-15·4]; p<0·0001). Risk of anaemia within 28 days was lower in patients in the artesunate-mefloquine group (71 [62%; 95% CI 52·2-70·6]) than in those in the chloroquine group (83 [75%; 65·6-82·5]; p=0·035). Gametocytaemia as detected by PCR for pks25 was present in 44 (86%) of 51 patients in the artesunate-mefloquine group and 41 (84%) of 49 patients in the chloroquine group at baseline, and in three (6%) of 49 patients and two (4%) of 48 patients, respectively, at day 7. Fever clearance was faster in the artesunate-mefloquine group (mean 11·5 h [95% CI 8·3-14·6]) than in the chloroquine group (14·8 h [11·7-17·8]; p=0·034). Bed occupancy was 2426 days per 1000 patients in the artesunate-mefloquine group versus 2828 days per 1000 patients in the chloroquine group (incidence rate ratio 0·858 [95% CI 0·812-0·906]; p<0·0001). One (<1%) patient in the artesunate-mefloquine group had a serious neuropsychiatric event regarded as probably related to study drug.
Interpretation: Artesunate-mefloquine is highly efficacious for treatment of uncomplicated P knowlesi malaria. The rapid therapeutic response of the drug offers significant advantages compared with chloroquine monotherapy and supports a unified treatment policy for artemisinin-based combination therapy for all Plasmodium species in co-endemic areas.
Funding: Malaysian Ministry of Health, Australian National Health and Medical Research Council, and Asia Pacific Malaria Elimination Network.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Artemisinin-based combination therapy for knowlesi malaria.Lancet Infect Dis. 2016 Feb;16(2):134-6. doi: 10.1016/S1473-3099(15)00475-2. Epub 2015 Nov 19. Lancet Infect Dis. 2016. PMID: 26603170 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
