Cytoskeletal changes induced by allosteric modulators of calcium-sensing receptor in esophageal epithelial cells
- PMID: 26603452
- PMCID: PMC4673644
- DOI: 10.14814/phy2.12616
Cytoskeletal changes induced by allosteric modulators of calcium-sensing receptor in esophageal epithelial cells
Abstract
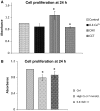
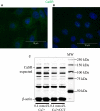
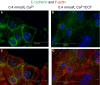
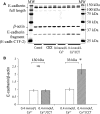
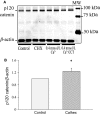
The calcium-sensing receptor (CaSR), a G-protein-coupled receptor, plays a role in glandular and fluid secretion in the gastrointestinal tract, and regulates differentiation and proliferation of epithelial cells. We examined the expression of CaSR in normal and pathological conditions of human esophagus and investigated the effect of a CaSR agonist, cinacalcet (CCT), and antagonist, calhex (CHX), on cell growth and cell-cell junctional proteins in primary cultures of porcine stratified squamous esophageal epithelium. We used immunohistochemistry and Western analysis to monitor expression of CaSR and cell-cell adhesion molecules, and MTT assay to monitor cell proliferation in cultured esophageal cells. CCT treatment significantly reduced proliferation, changed the cell shape from polygonal to spindle-like, and caused redistribution of E-cadherin and β-catenin from the cell membrane to the cytoplasm. Furthermore, it reduced expression of β-catenin by 35% (P < 0.02) and increased expression of a proteolysis cleavage fragment of E-cadherin, Ecad/CFT2, by 2.3 folds (P < 0.01). On the other hand, CHX treatment enhanced cell proliferation by 27% (P < 0.01), increased the expression of p120-catenin by 24% (P < 0.04), and of Rho, a GTPase involved in cytoskeleton remodeling, by 18% (P < 0.03). In conclusion, CaSR is expressed in normal esophagus as well as in Barrett's, esophageal adenocarcinoma, squamous cell carcinoma, and eosinophilic esophagitis. Long-term activation of CaSR with CCT disrupted the cadherin-catenin complex, induced cytoskeletal remodeling, actin fiber formation, and redistribution of CaSR to the nuclear area. These changes indicate a significant and complex role of CaSR in epithelial remodeling and barrier function of esophageal cells.
Keywords: Adherens junction; E‐cad/CTF2; calcimimetics; esophagus; stratified squamous epithelium.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures








References
-
- Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Haque S, Wang P, Brown K, et al. Characterization of esophageal submucosal glands in pig tissue and cultures. Dig. Dis. Sci. 2007;52:3054–3065. - PubMed
-
- Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, Brown KL, Hansen M, Butcher KF, et al. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clin. Immunol. 2013;148:265–278. - PubMed
-
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, et al. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2000;2:637–644. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

