Regulation of macrophage development and function in peripheral tissues
- PMID: 26603899
- PMCID: PMC4706379
- DOI: 10.1038/nri3920
Regulation of macrophage development and function in peripheral tissues
Abstract
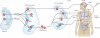
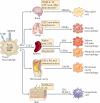
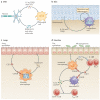
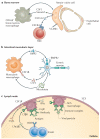
Macrophages are immune cells of haematopoietic origin that provide crucial innate immune defence and have tissue-specific functions in the regulation and maintenance of organ homeostasis. Recent studies of macrophage ontogeny, as well as transcriptional and epigenetic identity, have started to reveal the decisive role of the tissue stroma in the regulation of macrophage function. These findings suggest that most macrophages seed the tissues during embryonic development and functionally specialize in response to cytokines and metabolites that are released by the stroma and drive the expression of unique transcription factors. In this Review, we discuss how recent insights into macrophage ontogeny and macrophage-stroma interactions contribute to our understanding of the crosstalk that shapes macrophage function and the maintenance of organ integrity.
Figures
References
-
- Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. [This ImmGen consortia study shows that tissue macrophages have a distinct transcriptional profile that depends on the tissue in which they reside, highlighting the potential contribution of tissue imprinting to macrophage identity.] - PMC - PubMed
-
- Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. - PubMed
-
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. - PubMed
-
- Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

