STING: infection, inflammation and cancer
- PMID: 26603901
- PMCID: PMC5004891
- DOI: 10.1038/nri3921
STING: infection, inflammation and cancer
Abstract
The rapid detection of microbial agents is essential for the effective initiation of host defence mechanisms against infection. Understanding how cells detect cytosolic DNA to trigger innate immune gene transcription has important implications - not only for comprehending the immune response to pathogens but also for elucidating the causes of autoinflammatory disease involving the sensing of self-DNA and the generation of effective antitumour adaptive immunity. The discovery of the STING (stimulator of interferon genes)-controlled innate immune pathway, which mediates cytosolic DNA-induced signalling events, has recently provided important insights into these processes, opening the way for the development of novel immunization regimes, as well as therapies to treat autoinflammatory disease and cancer.
Conflict of interest statement
statement The author declares no competing interests.
Figures
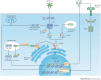
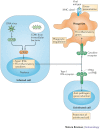
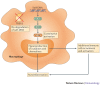
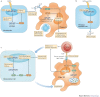
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

