Bovine milk-derived exosomes for drug delivery
- PMID: 26604130
- PMCID: PMC4706492
- DOI: 10.1016/j.canlet.2015.10.020
Bovine milk-derived exosomes for drug delivery
Abstract
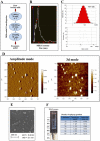
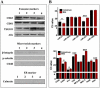
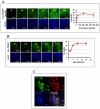
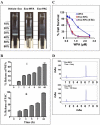
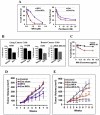
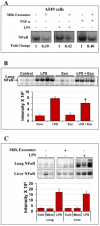
Exosomes are biological nanovesicles that are involved in cell-cell communication via the functionally-active cargo (such as miRNA, mRNA, DNA and proteins). Because of their nanosize, exosomes are explored as nanodevices for the development of new therapeutic applications. However, bulk, safe and cost-effective production of exosomes is not available. Here, we show that bovine milk can serve as a scalable source of exosomes that can act as a carrier for chemotherapeutic/chemopreventive agents. Drug-loaded exosomes showed significantly higher efficacy compared to free drug in cell culture studies and against lung tumor xenografts in vivo. Moreover, tumor targeting ligands such as folate increased cancer-cell targeting of the exosomes resulting in enhanced tumor reduction. Milk exosomes exhibited cross-species tolerance with no adverse immune and inflammatory response. Thus, we show the versatility of milk exosomes with respect to the cargo it can carry and ability to achieve tumor targetability. This is the first report to identify a biocompatible and cost-effective means of exosomes to enhance oral bioavailability, improve efficacy and safety of drugs.
Keywords: Chemopreventive agents; Chemotherapeutic drugs; Drug delivery; Milk-derived exosomes; Tumor-targeting.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. Journal of controlled release : official journal of the Controlled Release Society. 2012;160:117–134. - PubMed
-
- Ciruelos E, Jackisch C. Evaluating the role of nab-paclitaxel (Abraxane) in women with aggressive metastatic breast cancer. Expert review of anticancer therapy. 2014;14:511–521. - PubMed
-
- Saif MW. U.S. Food and Drug Administration approves paclitaxel protein-bound particles (Abraxane(R)) in combination with gemcitabine as first-line treatment of patients with metastatic pancreatic cancer. JOP : Journal of the pancreas. 2013;14:686–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

