Reply to Marchenko et al.: Flux analysis of GroEL-assisted protein folding/unfolding
- PMID: 26604310
- PMCID: PMC4687547
- DOI: 10.1073/pnas.1520474112
Reply to Marchenko et al.: Flux analysis of GroEL-assisted protein folding/unfolding
Conflict of interest statement
The authors declare no conflict of interest.
Figures
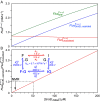
Comment on
-
Intrinsic unfoldase/foldase activity of the chaperonin GroEL directly demonstrated using multinuclear relaxation-based NMR.Proc Natl Acad Sci U S A. 2015 Jul 21;112(29):8817-23. doi: 10.1073/pnas.1510083112. Epub 2015 Jun 29. Proc Natl Acad Sci U S A. 2015. PMID: 26124125 Free PMC article.
-
Strict experimental evidence that apo-chaperonin GroEL does not accelerate protein folding, although it does accelerate one of its steps.Proc Natl Acad Sci U S A. 2015 Dec 15;112(50):E6831-2. doi: 10.1073/pnas.1517712112. Epub 2015 Nov 24. Proc Natl Acad Sci U S A. 2015. PMID: 26604318 Free PMC article. No abstract available.
References
-
- Marchenkov VV, et al. [The interaction of the GroEL chaperone with early kinetic intermediates of renaturing proteins inhibits the formation of their native structure] Biofizika. 2004;49(6):987–994. Russian. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

