PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density
- PMID: 26604311
- PMCID: PMC4687590
- DOI: 10.1073/pnas.1517045112
PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density
Abstract
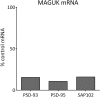
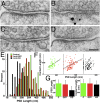
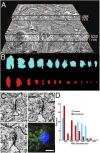
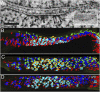
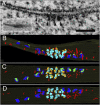
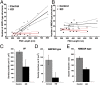
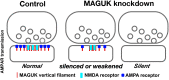
The postsynaptic density (PSD)-95 family of membrane-associated guanylate kinases (MAGUKs) are major scaffolding proteins at the PSD in glutamatergic excitatory synapses, where they maintain and modulate synaptic strength. How MAGUKs underlie synaptic strength at the molecular level is still not well understood. Here, we explore the structural and functional roles of MAGUKs at hippocampal excitatory synapses by simultaneous knocking down PSD-95, PSD-93, and synapse-associated protein (SAP)102 and combining electrophysiology and transmission electron microscopic (TEM) tomography imaging to analyze the resulting changes. Acute MAGUK knockdown greatly reduces synaptic transmission mediated by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) and N-methyl-d-aspartate receptors (NMDARs). This knockdown leads to a significant rise in the number of silent synapses, diminishes the size of PSDs without changes in pre- or postsynaptic membrane, and depletes the number of membrane-associated PSD-95-like vertical filaments and transmembrane structures, identified as AMPARs and NMDARs by EM tomography. The differential distribution of these receptor-like structures and dependence of their abundance on PSD size matches that of AMPARs and NMDARs in the hippocampal synapses. The loss of these structures following MAGUK knockdown tracks the reduction in postsynaptic AMPAR and NMDAR transmission, confirming the structural identities of these two types of receptors. These results demonstrate that MAGUKs are required for anchoring both types of glutamate receptors at the PSD and are consistent with a structural model where MAGUKs, corresponding to membrane-associated vertical filaments, are the essential structural proteins that anchor and organize both types of glutamate receptors and govern the overall molecular organization of the PSD.
Keywords: AMPAR; EM tomography; MAGUKs; NMDAR; knockdown.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290(5492):750–754. - PubMed
-
- Chen X, et al. Nanoscale Imaging of Protein Molecules at the Postsynaptic Density. Springer Science+Business Media; New York: 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

