Exploring the heat-induced structural changes of β-lactoglobulin -linoleic acid complex by fluorescence spectroscopy and molecular modeling techniques
- PMID: 26604382
- PMCID: PMC4648940
- DOI: 10.1007/s13197-015-1949-2
Exploring the heat-induced structural changes of β-lactoglobulin -linoleic acid complex by fluorescence spectroscopy and molecular modeling techniques
Abstract
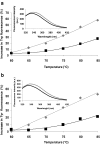
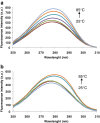
Linoleic acid (LA) is the precursor of bioactive oxidized linoleic acid metabolites and arachidonic acid, therefore is essential for human growth and plays an important role in good health in general. Because of the low water solubility and sensitivity to oxidation, new ways of LA delivery without compromising the sensory attributes of the enriched products are to be identified. The major whey protein, β-lactoglobulin (β-Lg), is a natural carrier for hydrophobic molecules. The thermal induced changes of the β-Lg-LA complex were investigated in the temperature range from 25 to 85 °C using fluorescence spectroscopy techniques in combination with molecular modeling study and the results were compared with those obtained for β-Lg. Experimental results indicated that, regardless of LA binding, the polypeptide chain rearrangements at temperatures higher than 75 °C lead to higher exposure of hydrophobic residues causing the increase of fluorescence intensity. Phase diagram indicated an all or none transition between two conformations. The LA surface involved in the interaction with β-Lg was about 497 Ǻ(2), indicating a good affinity between those two components even at high temperatures. Results obtained in this study provide important details about heat-induced changes in the conformation of β-Lg-LA complex. The thermal treatment at high temperature does not affect the LA binding and carrier functions of β-Lg.
Keywords: Fluorescence spectroscopy; Linoleic acid; Molecular modeling; Structural changes; β-lactoglobulin.
Figures




Similar articles
-
Self-assembled β-lactoglobulin-oleic acid and β-lactoglobulin-linoleic acid complexes with antitumor activities.J Dairy Sci. 2015 May;98(5):2898-907. doi: 10.3168/jds.2014-8993. Epub 2015 Mar 12. J Dairy Sci. 2015. PMID: 25771044
-
Research of carrying mechanism between β-lactoglobulin and linoleic acid: Effects on protein structure and oxidative stability of linoleic acid.Biochem Biophys Res Commun. 2025 Feb 2;747:151298. doi: 10.1016/j.bbrc.2025.151298. Epub 2025 Jan 8. Biochem Biophys Res Commun. 2025. PMID: 39799867
-
A comparison study of the interaction between β-lactoglobulin and retinol at two different conditions: spectroscopic and molecular modeling approaches.J Biomol Struct Dyn. 2015 Sep;33(9):1880-98. doi: 10.1080/07391102.2014.977351. Epub 2014 Nov 17. J Biomol Struct Dyn. 2015. PMID: 25402748
-
Invited review: beta-lactoglobulin: binding properties, structure, and function.J Dairy Sci. 2004 Apr;87(4):785-96. doi: 10.3168/jds.S0022-0302(04)73222-1. J Dairy Sci. 2004. PMID: 15259212 Review.
-
Bovine β-lactoglobulin/fatty acid complexes: binding, structural, and biological properties.Dairy Sci Technol. 2014;94(5):409-426. doi: 10.1007/s13594-014-0160-y. Epub 2014 Feb 27. Dairy Sci Technol. 2014. PMID: 25110551 Free PMC article. Review.
Cited by
-
Characterization of molecular structures of theaflavins and the interactions with bovine serum albumin.J Food Sci Technol. 2017 Oct;54(11):3421-3432. doi: 10.1007/s13197-017-2791-5. Epub 2017 Sep 12. J Food Sci Technol. 2017. PMID: 29051637 Free PMC article.
-
The Impact of AAPH-Induced Oxidation on the Functional and Structural Properties, and Proteomics of Arachin.Molecules. 2023 Aug 28;28(17):6277. doi: 10.3390/molecules28176277. Molecules. 2023. PMID: 37687106 Free PMC article.
-
Supercritical CO2 Fluid Extraction and Microencapsulation of Oil from Anchovy (Engraulis mordax) By-Products.Food Technol Biotechnol. 2024 Sep;62(3):302-313. doi: 10.17113/ftb.62.03.24.8336. Food Technol Biotechnol. 2024. PMID: 39497696 Free PMC article.
References
-
- Bergamo P., Luongo D., Miyamoto J., Cocca E., Kishino S., Ogawa J., Tanabe S., Rossi M. Immunomodulatory activity of a gut microbial metabolite of dietary linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, associated with improved antioxidant/detoxifying defences. J Funct Foods. 2014;II:192–202. doi: 10.1016/j.jff.2014.10.007. - DOI
-
- Bi S., Yan L., Pang B., Wang Y. Investigation of three flavonoids binding to bovine serum albumin using molecular fluorescence technique. J Lumin. 2012;132:132–140. doi: 10.1016/j.jlumin.2011.08.014. - DOI
-
- Buccioni A., Decandia M., Minieri S., Molle G., Cabiddu A. Lipid metabolism in the rumen: new insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim Feed Sci Technol. 2012;174:1–25. doi: 10.1016/j.anifeedsci.2012.02.009. - DOI
-
- Callis P. Binding phenomena and fluorescence quenching. I: descriptive quantum principles of fluorescence quenching using a supermolecule approach. J Mol Struct. 2014;1077:14–21. doi: 10.1016/j.molstruc.2014.04.050. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
