Stability and biological activity evaluations of PEGylated human basic fibroblast growth factor
- PMID: 26605215
- PMCID: PMC4616999
- DOI: 10.4103/2277-9175.164001
Stability and biological activity evaluations of PEGylated human basic fibroblast growth factor
Abstract
Background: Human basic fibroblast growth factor (hBFGF) is a heparin-binding growth factor and stimulates the proliferation of a wide variety of cells and tissues causing survival properties and its stability and biological activity improvements have received much attention.
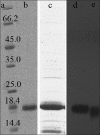
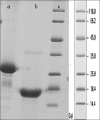
Materials and methods: In the present work, hBFGF produced by engineered Escherichia coli and purified by cation exchange and heparin affinity chromatography, was PEGylated under appropriate condition employing 10 kD polyethylene glycol. The PEGylated form was separated by size exclusion chromatography. Structural, biological activity, and stability evaluations were performed using Fourier transform infrared (FITR) spectroscopy, 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) assay and effect denaturing agent, respectively.
Results: FITR spectroscopy revealed that both PEGylated and native forms had the same structures. MTT assay showed that PEGyalated form had a 30% reduced biological activity. Fluorescence spectrophotometry indicated that the PEGylated form denatured at higher concentrations of guanidine HCl (1.2 M) compared with native, which denatured at 0.8 M guanidine HCl.
Conclusions: PEGylation of hBFGF makes it more stable against denaturing agent but reduces its bioactivity up to 30%.
Keywords: 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide assay; Fourier transform infrared spectroscopy; human basic fibroblast growth factor; polyethylene glycol methyl ether maleimide; stability.
Conflict of interest statement
Figures



Similar articles
-
Purification and modification by polyethylene glycol of a new human basic fibroblast growth factor mutant-hbFGF(Ser25,87,92).J Chromatogr A. 2007 Aug 17;1161(1-2):51-5. doi: 10.1016/j.chroma.2007.01.135. Epub 2007 Feb 8. J Chromatogr A. 2007. PMID: 17307188
-
Scaling up production of recombinant human basic fibroblast growth factor in an Escherichia coli BL21(DE3) plysS strain and evaluation of its pro-wound healing efficacy.Front Pharmacol. 2024 Feb 5;14:1279516. doi: 10.3389/fphar.2023.1279516. eCollection 2023. Front Pharmacol. 2024. PMID: 38375209 Free PMC article.
-
Site-directed PEGylation of human basic fibroblast growth factor.Protein Expr Purif. 2006 Jul;48(1):24-7. doi: 10.1016/j.pep.2006.02.002. Epub 2006 Feb 28. Protein Expr Purif. 2006. PMID: 16545577
-
Purification of pegylated proteins.Methods Biochem Anal. 2011;54:339-62. doi: 10.1002/9780470939932.ch14. Methods Biochem Anal. 2011. PMID: 21954785 Review.
-
The impact of PEGylation on biological therapies.BioDrugs. 2008;22(5):315-29. doi: 10.2165/00063030-200822050-00004. BioDrugs. 2008. PMID: 18778113 Review.
Cited by
-
Fibroblast growth factor 2 dimer with superagonist in vitro activity improves granulation tissue formation during wound healing.Biomaterials. 2016 Mar;81:157-168. doi: 10.1016/j.biomaterials.2015.12.003. Epub 2015 Dec 15. Biomaterials. 2016. PMID: 26731578 Free PMC article.
References
-
- Szymkowski DE. Creating the next generation of protein therapeutics through rational drug design. Curr Opin Drug Discov Devel. 2005;8:590–600. - PubMed
-
- Jevsevar S, Kunstelj M, Porekar VG. PEGylation of therapeutic proteins. Biotechnol J. 2010;5:113–28. - PubMed
-
- Mateo C, Lombardero J, Moreno E, Morales A, Bombino G, Coloma J, et al. Removal of amphipathic epitopes from genetically engineered antibodies: Production of modified immunoglobulins with reduced immunogenicity. Hybridoma. 2000;19:463–71. - PubMed
-
- Walsh G. Second-generation biopharmaceuticals. Eur J Pharm Biopharm. 2004;58:185–96. - PubMed
-
- Tian H, Guo Y, Gao X, Yao W. PEGylation enhancement of pH stability of uricase via inhibitive tetramer dissociation. J Pharm Pharmacol. 2013;65:53–63. - PubMed

