Mitochondrial DNA Depletion in Respiratory Chain-Deficient Parkinson Disease Neurons
- PMID: 26605748
- PMCID: PMC4819690
- DOI: 10.1002/ana.24571
Mitochondrial DNA Depletion in Respiratory Chain-Deficient Parkinson Disease Neurons
Abstract
Objective: To determine the extent of respiratory chain abnormalities and investigate the contribution of mtDNA to the loss of respiratory chain complexes (CI-IV) in the substantia nigra (SN) of idiopathic Parkinson disease (IPD) patients at the single-neuron level.
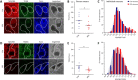
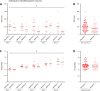
Methods: Multiple-label immunofluorescence was applied to postmortem sections of 10 IPD patients and 10 controls to quantify the abundance of CI-IV subunits (NDUFB8 or NDUFA13, SDHA, UQCRC2, and COXI) and mitochondrial transcription factors (TFAM and TFB2M) relative to mitochondrial mass (porin and GRP75) in dopaminergic neurons. To assess the involvement of mtDNA in respiratory chain deficiency in IPD, SN neurons, isolated with laser-capture microdissection, were assayed for mtDNA deletions, copy number, and presence of transcription/replication-associated 7S DNA employing a triplex real-time polymerase chain reaction (PCR) assay.
Results: Whereas mitochondrial mass was unchanged in single SN neurons from IPD patients, we observed a significant reduction in the abundances of CI and II subunits. At the single-cell level, CI and II deficiencies were correlated in patients. The CI deficiency concomitantly occurred with low abundances of the mtDNA transcription factors TFAM and TFB2M, which also initiate transcription-primed mtDNA replication. Consistent with this, real-time PCR analysis revealed fewer transcription/replication-associated mtDNA molecules and an overall reduction in mtDNA copy number in patients. This effect was more pronounced in single IPD neurons with severe CI deficiency.
Interpretation: Respiratory chain dysfunction in IPD neurons not only involves CI, but also extends to CII. These deficiencies are possibly a consequence of the interplay between nDNA and mtDNA-encoded factors mechanistically connected via TFAM.
© 2016 The Authors. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association.
Figures




References
-
- Schapira AH. Mitochondrial pathology in Parkinson's disease. Mt Sinai J Med 2011;78:872–881. - PubMed
-
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine‐analog synthesis. Science 1983;219:979–980. - PubMed
-
- Schapira AH, Cooper JM, Dexter D, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet 1989;1:1269. - PubMed
-
- Kumar KR, Djarmati‐Westenberger A, Grunewald A. Genetics of Parkinson's disease. Semin Neurol 2011;31:433–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/L016451/1/MRC_/Medical Research Council/United Kingdom
- G1100540/MRC_/Medical Research Council/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G0900652/MRC_/Medical Research Council/United Kingdom
- 096919/WT_/Wellcome Trust/United Kingdom
- G0400074/MRC_/Medical Research Council/United Kingdom
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
- G0700718/MRC_/Medical Research Council/United Kingdom
- G0800674/MRC_/Medical Research Council/United Kingdom
- G906919/WT_/Wellcome Trust/United Kingdom
- G0502157/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

