Association of Baseline Visual Acuity and Retinal Thickness With 1-Year Efficacy of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema
- PMID: 26605836
- PMCID: PMC5567793
- DOI: 10.1001/jamaophthalmol.2015.4599
Association of Baseline Visual Acuity and Retinal Thickness With 1-Year Efficacy of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema
Erratum in
-
Error in Table.JAMA Ophthalmol. 2016 Apr;134(4):469. doi: 10.1001/jamaophthalmol.2016.0428. JAMA Ophthalmol. 2016. PMID: 26967962 No abstract available.
Abstract
Importance: Comparisons of the relative effect of 3 anti-vascular endothelial growth factor agents to treat diabetic macular edema warrant further assessment.
Objective: To provide additional outcomes from a randomized trial evaluating 3 anti-vascular endothelial growth factor agents for diabetic macular edema within subgroups based on baseline visual acuity (VA) and central subfield thickness (CST) as evaluated on optical coherence tomography.
Design, setting, and participants: Post hoc exploratory analyses were conducted of randomized trial data on 660 adults with diabetic macular edema and decreased VA (Snellen equivalent, approximately 20/32 to 20/320). The original study was conducted between August 22, 2012, and August 28, 2013. Analysis was conducted from January 7 to June 2, 2015.
Interventions: Repeated 0.05-mL intravitreous injections of 2.0 mg of aflibercept (224 eyes), 1.25 mg of bevacizumab (218 eyes), or 0.3 mg of ranibizumab (218 eyes) as needed per protocol.
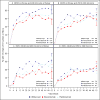
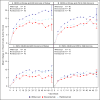
Main outcomes and measures: One-year VA and CST outcomes within prespecified subgroups based on both baseline VA and CST thresholds, defined as worse (20/50 or worse) or better (20/32 to 20/40) VA and thicker (≥400 µm) or thinner (250 to 399 µm) CST.
Results: In the subgroup with worse baseline VA (n = 305), irrespective of baseline CST, aflibercept showed greater improvement than bevacizumab or ranibizumab for several VA outcomes. In the subgroup with better VA and thinner CST at baseline (61-73 eyes across 3 treatment groups), VA outcomes showed little difference between groups; mean change was +7.2, +8.4, and +7.6 letters in the aflibercept, bevacizumab, and ranibizumab groups, respectively. However, in the subgroup with better VA and thicker CST at baseline (31-43 eyes), there was a suggestion of worse VA outcomes in the bevacizumab group; mean change from baseline to 1 year was +9.5, +5.4, and +9.5 letters in the aflibercept, bevacizumab, and ranibizumab groups, respectively, and VA letter score was greater than 84 (approximately 20/20) in 21 of 33 (64%), 7 of 31 (23%), and 21 of 43 (49%) eyes, respectively. The adjusted differences and 95% CIs were 39% (17% to 60%) for aflibercept vs bevacizumab, 25% (5% to 46%) for ranibizumab vs bevacizumab, and 13% (-8% to 35%) for aflibercept vs ranibizumab.
Conclusions and relevance: These post hoc secondary findings suggest that for eyes with better initial VA and thicker CST, some VA outcomes may be worse in the bevacizumab group than in the aflibercept and ranibizumab groups. Given the exploratory nature of these analyses and the small sample size within subgroups, caution is suggested when using the data to guide treatment considerations for patients.
Trial registration: clinicaltrials.gov Identifier: NCT01627249.
Figures
Comment in
-
Drilling Deeper for Treatment Choices in Diabetic Macular Edema.JAMA Ophthalmol. 2016 Feb;134(2):135-6. doi: 10.1001/jamaophthalmol.2015.4652. JAMA Ophthalmol. 2016. PMID: 26606738 Free PMC article. No abstract available.
References
-
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. - PubMed
-
- Aiello LP, Edwards AR, Beck RW, Bressler NM, Davis MD, Ferris F, Glassman AR, Ip MS, Miller KM, for the Diabetic Retinopathy Clinical Research Network Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2010;117:946–53. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

