M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia pneumoniae
- PMID: 26606059
- PMCID: PMC4659546
- DOI: 10.1371/journal.pone.0143593
M2 Polarization of Human Macrophages Favors Survival of the Intracellular Pathogen Chlamydia pneumoniae
Abstract
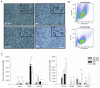
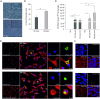
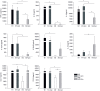
Intracellular pathogens have developed various strategies to escape immunity to enable their survival in host cells, and many bacterial pathogens preferentially reside inside macrophages, using diverse mechanisms to penetrate their defenses and to exploit their high degree of metabolic diversity and plasticity. Here, we characterized the interactions of the intracellular pathogen Chlamydia pneumoniae with polarized human macrophages. Primary human monocytes were pre-differentiated with granulocyte macrophage colony-stimulating factor or macrophage colony-stimulating factor for 7 days to yield M1-like and M2-like macrophages, which were further treated with interferon-γ and lipopolysaccharide or with interleukin-4 for 48 h to obtain fully polarized M1 and M2 macrophages. M1 and M2 cells exhibited distinct morphology with round or spindle-shaped appearance for M1 and M2, respectively, distinct surface marker profiles, as well as different cytokine and chemokine secretion. Macrophage polarization did not influence uptake of C. pneumoniae, since comparable copy numbers of chlamydial DNA were detected in M1 and M2 at 6 h post infection, but an increase in chlamydial DNA over time indicating proliferation was only observed in M2. Accordingly, 72±5% of M2 vs. 48±7% of M1 stained positive for chlamydial lipopolysaccharide, with large perinuclear inclusions in M2 and less clearly bordered inclusions for M1. Viable C. pneumoniae was present in lysates from M2, but not from M1 macrophages. The ability of M1 to restrict chlamydial replication was not observed in M1-like macrophages, since chlamydial load showed an equal increase over time for M1-like and M2-like macrophages. Our findings support the importance of macrophage polarization for the control of intracellular infection, and show that M2 are the preferred survival niche for C. pneumoniae. M1 did not allow for chlamydial proliferation, but failed to completely eliminate chlamydial infection, giving further evidence for the ability of C. pneumoniae to evade cellular defense and to persist in human macrophages.
Conflict of interest statement
Figures



References
-
- Kern JM, Maass V, Maass M. Molecular pathogenesis of chronic Chlamydia pneumoniae infection: a brief overview. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2009;15(1):36–41. 10.1111/j.1469-0691.2008.02631.x . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

