Spatio-Temporal Regulation of Rac1 Mobility by Actin Islands
- PMID: 26606145
- PMCID: PMC4659588
- DOI: 10.1371/journal.pone.0143753
Spatio-Temporal Regulation of Rac1 Mobility by Actin Islands
Abstract
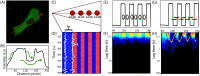
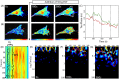
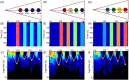
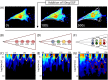
Rho GTPases play important roles in many aspects of cell migration, including polarity establishment and organizing actin cytoskeleton. In particular, the Rho GTPase Rac1 has been associated with the generation of protrusions at leading edge of migrating cells. Previously we showed the mobility of Rac1 molecules is not uniform throughout a migrating cell (Hinde E et. al. PNAS 2013). Specifically, the closer a Rac1 molecule is to the leading edge, the slower the molecule diffuses. Because actin-bound Rac1 diffuses slower than unbound Rac1, we hypothesized that regions of high actin concentration, called "actin islands", act as diffusive traps and are responsible for the non-uniform diffusion observed in vivo. Here, in silico model simulations demonstrate that equally spaced actin islands can regulate the time scale for Rac1 diffusion in a manner consistent with data from live-cell imaging experiments. Additionally, we find this mechanism is robust; different patterns of Rac1 mobility can be achieved by changing the actin islands' positions or their affinity for Rac1.
Conflict of interest statement
Figures
Similar articles
-
FilGAP and its close relatives: a mediator of Rho-Rac antagonism that regulates cell morphology and migration.Biochem J. 2013 Jul 1;453(1):17-25. doi: 10.1042/BJ20130290. Biochem J. 2013. PMID: 23763313 Review.
-
Spatio-temporal co-ordination of RhoA, Rac1 and Cdc42 activation during prototypical edge protrusion and retraction dynamics.Sci Rep. 2016 Feb 25;6:21901. doi: 10.1038/srep21901. Sci Rep. 2016. PMID: 26912264 Free PMC article.
-
A dual role for Rac1 GTPases in the regulation of cell motility.J Cell Sci. 2012 Jan 15;125(Pt 2):387-98. doi: 10.1242/jcs.089680. Epub 2012 Feb 2. J Cell Sci. 2012. PMID: 22302991
-
PLEKHG3 enhances polarized cell migration by activating actin filaments at the cell front.Proc Natl Acad Sci U S A. 2016 Sep 6;113(36):10091-6. doi: 10.1073/pnas.1604720113. Epub 2016 Aug 23. Proc Natl Acad Sci U S A. 2016. PMID: 27555588 Free PMC article.
-
Crosstalk between Rac1-mediated actin regulation and ROS production.Free Radic Biol Med. 2018 Feb 20;116:101-113. doi: 10.1016/j.freeradbiomed.2018.01.008. Epub 2018 Jan 10. Free Radic Biol Med. 2018. PMID: 29330095 Review.
Cited by
-
Spatial and temporal dynamics of RhoA activities of single breast tumor cells in a 3D environment revealed by a machine learning-assisted FRET technique.Exp Cell Res. 2022 Jan 15;410(2):112939. doi: 10.1016/j.yexcr.2021.112939. Epub 2021 Nov 20. Exp Cell Res. 2022. PMID: 34813733 Free PMC article.
References
-
- Burridge K, Wennerberg K (2004) Rho and Rac Take Center Stage. Cell 116: 167–179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

