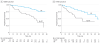Failure-Free Survival and Radiotherapy in Patients With Newly Diagnosed Nonmetastatic Prostate Cancer: Data From Patients in the Control Arm of the STAMPEDE Trial
- PMID: 26606329
- PMCID: PMC4789485
- DOI: 10.1001/jamaoncol.2015.4350
Failure-Free Survival and Radiotherapy in Patients With Newly Diagnosed Nonmetastatic Prostate Cancer: Data From Patients in the Control Arm of the STAMPEDE Trial
Erratum in
-
Error in Corresponding Author Address.JAMA Oncol. 2016 Feb;2(2):279. doi: 10.1001/jamaoncol.2016.0032. JAMA Oncol. 2016. PMID: 26869005 No abstract available.
Abstract
Importance: The natural history of patients with newly diagnosed high-risk nonmetastatic (M0) prostate cancer receiving hormone therapy (HT) either alone or with standard-of-care radiotherapy (RT) is not well documented. Furthermore, no clinical trial has assessed the role of RT in patients with node-positive (N+) M0 disease. The STAMPEDE Trial includes such individuals, allowing an exploratory multivariate analysis of the impact of radical RT.
Objective: To describe survival and the impact on failure-free survival of RT by nodal involvement in these patients.
Design, setting, and participants: Cohort study using data collected for patients allocated to the control arm (standard-of-care only) of the STAMPEDE Trial between October 5, 2005, and May 1, 2014. Outcomes are presented as hazard ratios (HRs) with 95% CIs derived from adjusted Cox models; survival estimates are reported at 2 and 5 years. Participants were high-risk, hormone-naive patients with newly diagnosed M0 prostate cancer starting long-term HT for the first time. Radiotherapy is encouraged in this group, but mandated for patients with node-negative (N0) M0 disease only since November 2011.
Exposures: Long-term HT either alone or with RT, as per local standard. Planned RT use was recorded at entry.
Main outcomes and measures: Failure-free survival (FFS) and overall survival.
Results: A total of 721 men with newly diagnosed M0 disease were included: median age at entry, 66 (interquartile range [IQR], 61-72) years, median (IQR) prostate-specific antigen level of 43 (18-88) ng/mL. There were 40 deaths (31 owing to prostate cancer) with 17 months' median follow-up. Two-year survival was 96% (95% CI, 93%-97%) and 2-year FFS, 77% (95% CI, 73%-81%). Median (IQR) FFS was 63 (26 to not reached) months. Time to FFS was worse in patients with N+ disease (HR, 2.02 [95% CI, 1.46-2.81]) than in those with N0 disease. Failure-free survival outcomes favored planned use of RT for patients with both N0M0 (HR, 0.33 [95% CI, 0.18-0.61]) and N+M0 disease (HR, 0.48 [95% CI, 0.29-0.79]).
Conclusions and relevance: Survival for men entering the cohort with high-risk M0 disease was higher than anticipated at study inception. These nonrandomized data were consistent with previous trials that support routine use of RT with HT in patients with N0M0 disease. Additionally, the data suggest that the benefits of RT extend to men with N+M0 disease.
Trial registration: clinicaltrials.gov Identifier: NCT00268476; ISRCTN78818544.
Figures
Comment in
-
Pelvis Plus Prostate Radiation Therapy and the Risk of Death in Men With Newly Diagnosed Node-Positive Prostate Cancer.JAMA Oncol. 2016 Mar;2(3):357-8. doi: 10.1001/jamaoncol.2015.4342. JAMA Oncol. 2016. PMID: 26605852 No abstract available.
-
Re: Failure-Free Survival and Radiotherapy in Patients with Newly Diagnosed Nonmetastatic Prostate Cancer: Data from Patients in the Control Arm of the STAMPEDE Trial.Eur Urol. 2016 Aug;70(2):398-9. doi: 10.1016/j.eururo.2016.03.065. Epub 2016 Apr 18. Eur Urol. 2016. PMID: 27353963 No abstract available.
References
-
- James ND, Sydes MR, Clarke NW, et al. STAMPEDE: Systemic Therapy for Advancing or Metastatic Prostate Cancer—a multi-arm multi-stage randomised controlled trial. Clin Oncol (R Coll Radiol) 2008;20(8):577–581. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 12459/CRUK_/Cancer Research UK/United Kingdom
- 10588/CRUK_/Cancer Research UK/United Kingdom
- 3804/CRUK_/Cancer Research UK/United Kingdom
- 12518/CRUK_/Cancer Research UK/United Kingdom
- MR/K006584/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/25/MRC_/Medical Research Council/United Kingdom
- 19727/CRUK_/Cancer Research UK/United Kingdom
- MC_UU_12023/6/MRC_/Medical Research Council/United Kingdom
- 13239/CRUK_/Cancer Research UK/United Kingdom
- A3804/CRUK_/Cancer Research UK/United Kingdom
- PG12-49/PCUK_/Prostate Cancer UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous