Brief Report: Patterns and Secular Trends in Use of Immunomodulatory Agents During Pregnancy in Women With Rheumatic Conditions
- PMID: 26606742
- PMCID: PMC4848128
- DOI: 10.1002/art.39521
Brief Report: Patterns and Secular Trends in Use of Immunomodulatory Agents During Pregnancy in Women With Rheumatic Conditions
Abstract
Objective: To describe patterns and secular trends in the use of immunomodulatory agents in pregnant women with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS).
Methods: We identified a cohort of women with SLE, RA, PsA, or AS enrolled in public (Medicaid, 2001-2010) or private (Optum Clinformatics, 2004-2012) health insurance, and we included women filling prescriptions for immunomodulatory agents (including steroids, nonbiologic disease-modifying agents, and biologic agents) in the 3-month period immediately prior to their pregnancies. The proportion of women continuing or discontinuing individual agents during pregnancy was reported. Annual prescription fill rates, estimated after accounting for patient characteristics and random variability from year to year in mixed-effects regression models, were used to conduct time trends analysis.
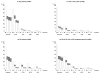
Results: We included 2,645 women being treated with immunomodulatory agents prior to pregnancy. More women with PsA or AS stopped filling prescriptions for immunomodulatory agents during pregnancy (61%) than women with SLE (26%) or women with RA (34.5%). From the first to the third trimester, the proportions of women filling prescriptions for immunomodulatory agents decreased across all indications. Overall, steroids and hydroxychloroquine were the most frequently used agents in pregnancy (48.4% and 27.1%, respectively). The rates (reported per 100 deliveries in our cohort) for steroid prescription fills during pregnancy decreased significantly from 54.4 in 2001 to 42.4 in 2012, while rates for biologic agents increased from 5.1 in 2001 to 16.6 in 2012 (P < 0.001 for both trends).
Conclusion: Steroids and hydroxychloroquine remain the most widely prescribed treatment options in pregnancy, but the use of biologic agents is becoming increasingly common.
© 2016, American College of Rheumatology.
Conflict of interest statement
Dr. Huybrechts is supported by a career development award from the National Institute of Mental Health (K01 MH099141). Dr. Bateman is supported by a career development award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the NIH (K08HD075831). Dr. Hernandez-Diaz is supported by the NIH grant R01 MH100216 and has consulted for AstraZeneca (London, UK) for unrelated projects. Dr. Kim is supported by the NIH grant K23 AR059677. She reports receiving research support from Pfizer, AstraZeneca and Lilly on unrelated projects. The other authors declare no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Ostensen M, Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983;26(9):1155–9. - PubMed
-
- Ostensen M. The effect of pregnancy on ankylosing spondylitis, psoriatic arthritis, and juvenile rheumatoid arthritis. Am J Reprod Immunol (New York, NY : 1989) 1992;28(3–4):235–7. - PubMed
-
- Barrett JH, Brennan P, Fiddler M, Silman AJ. Does rheumatoid arthritis remit during pregnancy and relapse postpartum? Results from a nationwide study in the United Kingdom performed prospectively from late pregnancy. Arthritis Rheum. 1999;42(6):1219–27. - PubMed
-
- de Man Y, Hazes JM, van der Heide H, Willemsen S, de Groot C, EAS, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60(11):3196–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

