Peripheral Adenosine A3 Receptor Activation Causes Regulated Hypothermia in Mice That Is Dependent on Central Histamine H1 Receptors
- PMID: 26606937
- PMCID: PMC4746492
- DOI: 10.1124/jpet.115.229872
Peripheral Adenosine A3 Receptor Activation Causes Regulated Hypothermia in Mice That Is Dependent on Central Histamine H1 Receptors
Abstract
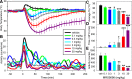
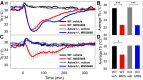
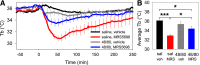
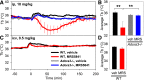
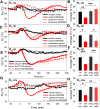
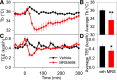
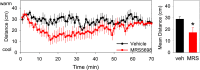
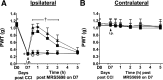
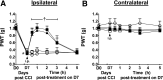
Adenosine can induce hypothermia, as previously demonstrated for adenosine A1 receptor (A1AR) agonists. Here we use the potent, specific A3AR agonists MRS5698, MRS5841, and MRS5980 to show that adenosine also induces hypothermia via the A3AR. The hypothermic effect of A3AR agonists is independent of A1AR activation, as the effect was fully intact in mice lacking A1AR but abolished in mice lacking A3AR. A3AR agonist-induced hypothermia was attenuated by mast cell granule depletion, demonstrating that the A3AR hypothermia is mediated, at least in part, via mast cells. Central agonist dosing had no clear hypothermic effect, whereas peripheral dosing of a non-brain-penetrant agonist caused hypothermia, suggesting that peripheral A3AR-expressing cells drive the hypothermia. Mast cells release histamine, and blocking central histamine H1 (but not H2 or H4) receptors prevented the hypothermia. The hypothermia was preceded by hypometabolism and mice with hypothermia preferred a cooler environmental temperature, demonstrating that the hypothermic state is a coordinated physiologic response with a reduced body temperature set point. Importantly, hypothermia is not required for the analgesic effects of A3AR agonists, which occur with lower agonist doses. These results support a mechanistic model for hypothermia in which A3AR agonists act on peripheral mast cells, causing histamine release, which stimulates central histamine H1 receptors to induce hypothermia. This mechanism suggests that A3AR agonists will probably not be useful for clinical induction of hypothermia.
U.S. Government work not protected by U.S. copyright.
Figures
Similar articles
-
Hypothermia in mouse is caused by adenosine A1 and A3 receptor agonists and AMP via three distinct mechanisms.Neuropharmacology. 2017 Mar 1;114:101-113. doi: 10.1016/j.neuropharm.2016.11.026. Epub 2016 Nov 30. Neuropharmacology. 2017. PMID: 27914963 Free PMC article.
-
Activation of adenosine A2A or A2B receptors causes hypothermia in mice.Neuropharmacology. 2018 Sep 1;139:268-278. doi: 10.1016/j.neuropharm.2018.02.035. Epub 2018 Mar 13. Neuropharmacology. 2018. PMID: 29548686 Free PMC article.
-
Profound hypothermia after adenosine kinase inhibition in A1AR-deficient mice suggests a receptor-independent effect of intracellular adenosine.Pflugers Arch. 2017 Feb;469(2):339-347. doi: 10.1007/s00424-016-1925-3. Epub 2016 Dec 14. Pflugers Arch. 2017. PMID: 27975140
-
Uncovering the Mechanisms of Adenosine Receptor-Mediated Pain Control: Focus on the A3 Receptor Subtype.Int J Mol Sci. 2021 Jul 26;22(15):7952. doi: 10.3390/ijms22157952. Int J Mol Sci. 2021. PMID: 34360719 Free PMC article. Review.
-
Adenosine A3 Receptor: From Molecular Signaling to Therapeutic Strategies for Heart Diseases.Int J Mol Sci. 2024 May 25;25(11):5763. doi: 10.3390/ijms25115763. Int J Mol Sci. 2024. PMID: 38891948 Free PMC article. Review.
Cited by
-
Ocular Purine Receptors as Drug Targets in the Eye.J Ocul Pharmacol Ther. 2016 Oct;32(8):534-547. doi: 10.1089/jop.2016.0090. Epub 2016 Aug 30. J Ocul Pharmacol Ther. 2016. PMID: 27574786 Free PMC article. Review.
-
A binding kinetics study of human adenosine A3 receptor agonists.Biochem Pharmacol. 2018 Jul;153:248-259. doi: 10.1016/j.bcp.2017.12.026. Epub 2018 Jan 3. Biochem Pharmacol. 2018. PMID: 29305857 Free PMC article.
-
Structure-Based Scaffold Repurposing for G Protein-Coupled Receptors: Transformation of Adenosine Derivatives into 5HT2B/5HT2C Serotonin Receptor Antagonists.J Med Chem. 2016 Dec 22;59(24):11006-11026. doi: 10.1021/acs.jmedchem.6b01183. Epub 2016 Dec 9. J Med Chem. 2016. PMID: 27933810 Free PMC article.
-
Purinergic Signaling in Mast Cell Degranulation and Asthma.Front Pharmacol. 2017 Dec 22;8:947. doi: 10.3389/fphar.2017.00947. eCollection 2017. Front Pharmacol. 2017. PMID: 29311944 Free PMC article. Review.
-
Melanotan II causes hypothermia in mice by activation of mast cells and stimulation of histamine 1 receptors.Am J Physiol Endocrinol Metab. 2018 Sep 1;315(3):E357-E366. doi: 10.1152/ajpendo.00024.2018. Epub 2018 May 29. Am J Physiol Endocrinol Metab. 2018. PMID: 29812984 Free PMC article.
References
-
- Arrich J, Holzer M, Havel C, Müllner M, Herkner H. (2012) Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 9:CD004128. - PubMed
-
- Auchampach JA, Rizvi A, Qiu Y, Tang XL, Maldonado C, Teschner S, Bolli R. (1997) Selective activation of A3 adenosine receptors with N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide protects against myocardial stunning and infarction without hemodynamic changes in conscious rabbits. Circ Res 80:800–809. - PubMed
-
- Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, et al. TOBY Study Group (2014) Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med 371:140–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

