Targeted Application of Human Genetic Variation Can Improve Red Blood Cell Production from Stem Cells
- PMID: 26607381
- PMCID: PMC4707983
- DOI: 10.1016/j.stem.2015.09.015
Targeted Application of Human Genetic Variation Can Improve Red Blood Cell Production from Stem Cells
Abstract
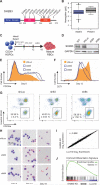
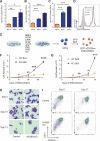
Multipotent and pluripotent stem cells are potential sources for cell and tissue replacement therapies. For example, stem cell-derived red blood cells (RBCs) are a potential alternative to donated blood, but yield and quality remain a challenge. Here, we show that application of insight from human population genetic studies can enhance RBC production from stem cells. The SH2B3 gene encodes a negative regulator of cytokine signaling and naturally occurring loss-of-function variants in this gene increase RBC counts in vivo. Targeted suppression of SH2B3 in primary human hematopoietic stem and progenitor cells enhanced the maturation and overall yield of in-vitro-derived RBCs. Moreover, inactivation of SH2B3 by CRISPR/Cas9 genome editing in human pluripotent stem cells allowed enhanced erythroid cell expansion with preserved differentiation. Our findings therefore highlight the potential for combining human genome variation studies with genome editing approaches to improve cell and tissue production for regenerative medicine.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
References
-
- Abkowitz JL, Sabo KM, Nakamoto B, Blau CA, Martin FH, Zsebo KM, Papayannopoulou T. Diamond-blackfan anemia: in vitro response of erythroid progenitors to the ligand for c-kit. Blood. 1991;78:2198–2202. - PubMed
-
- d'Onofrio G, Chirillo R, Zini G, Caenaro G, Tommasi M, Micciulli G. Simultaneous measurement of reticulocyte and red blood cell indices in healthy subjects and patients with microcytic and macrocytic anemia. Blood. 1995;85:818–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
