Navigating the bone marrow niche: translational insights and cancer-driven dysfunction
- PMID: 26607387
- PMCID: PMC4947935
- DOI: 10.1038/nrrheum.2015.160
Navigating the bone marrow niche: translational insights and cancer-driven dysfunction
Abstract
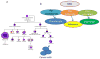
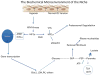
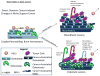
The bone marrow niche consists of stem and progenitor cells destined to become mature cells such as haematopoietic elements, osteoblasts or adipocytes. Marrow cells, influenced by endocrine, paracrine and autocrine factors, ultimately function as a unit to regulate bone remodelling and haematopoiesis. Current evidence highlights that the bone marrow niche is not merely an anatomic compartment; rather, it integrates the physiology of two distinct organ systems, the skeleton and the marrow. The niche has a hypoxic microenvironment that maintains quiescent haematopoietic stem cells (HSCs) and supports glycolytic metabolism. In response to biochemical cues and under the influence of neural, hormonal, and biochemical factors, marrow stromal elements, such as mesenchymal stromal cells (MSCs), differentiate into mature, functioning cells. However, disruption of the niche can affect cellular differentiation, resulting in disorders ranging from osteoporosis to malignancy. In this Review, we propose that the niche reflects the vitality of two tissues - bone and blood - by providing a unique environment for stem and stromal cells to flourish while simultaneously preventing disproportionate proliferation, malignant transformation or loss of the multipotent progenitors required for healing, functional immunity and growth throughout an organism's lifetime. Through a fuller understanding of the complexity of the niche in physiologic and pathologic states, the successful development of more-effective therapeutic approaches to target the niche and its cellular components for the treatment of rheumatic, endocrine, neoplastic and metabolic diseases becomes achievable.
Conflict of interest statement
The authors declare no competing interests.
Figures



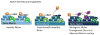
References
-
- Bianco P. ‘Mesenchymal’ stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. - PubMed
-
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. - PubMed
-
- Miguel MP, De Alcaina Y, Maza DS. de la & Lopez-Iglesias, P. Cell metabolism under microenvironmental low oxygen tension levels in stemness, proliferation and pluripotency. Curr Mol Med. 2015 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

