STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity
- PMID: 26607445
- PMCID: PMC4687570
- DOI: 10.1073/pnas.1512832112
STING activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity
Abstract
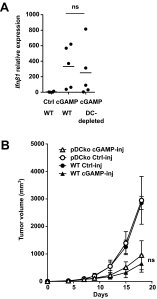
Spontaneous CD8 T-cell responses occur in growing tumors but are usually poorly effective. Understanding the molecular and cellular mechanisms that drive these responses is of major interest as they could be exploited to generate a more efficacious antitumor immunity. As such, stimulator of IFN genes (STING), an adaptor molecule involved in cytosolic DNA sensing, is required for the induction of antitumor CD8 T responses in mouse models of cancer. Here, we find that enforced activation of STING by intratumoral injection of cyclic dinucleotide GMP-AMP (cGAMP), potently enhanced antitumor CD8 T responses leading to growth control of injected and contralateral tumors in mouse models of melanoma and colon cancer. The ability of cGAMP to trigger antitumor immunity was further enhanced by the blockade of both PD1 and CTLA4. The STING-dependent antitumor immunity, either induced spontaneously in growing tumors or induced by intratumoral cGAMP injection was dependent on type I IFNs produced in the tumor microenvironment. In response to cGAMP injection, both in the mouse melanoma model and an ex vivo model of cultured human melanoma explants, the principal source of type I IFN was not dendritic cells, but instead endothelial cells. Similarly, endothelial cells but not dendritic cells were found to be the principal source of spontaneously induced type I IFNs in growing tumors. These data identify an unexpected role of the tumor vasculature in the initiation of CD8 T-cell antitumor immunity and demonstrate that tumor endothelial cells can be targeted for immunotherapy of melanoma.
Keywords: CD8 T cells; STING; antitumor immunity; tumor endothelial cells; type I IFNs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Valmori D, et al. Frequent cytolytic T-cell responses to peptide MAGE-A10(254-262) in melanoma. Cancer Res. 2001;61(2):509–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

