Amphibian chytridiomycosis: a review with focus on fungus-host interactions
- PMID: 26607488
- PMCID: PMC4660679
- DOI: 10.1186/s13567-015-0266-0
Amphibian chytridiomycosis: a review with focus on fungus-host interactions
Abstract
Amphibian declines and extinctions are emblematic for the current sixth mass extinction event. Infectious drivers of these declines include the recently emerged fungal pathogens Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans (Chytridiomycota). The skin disease caused by these fungi is named chytridiomycosis and affects the vital function of amphibian skin. Not all amphibians respond equally to infection and host responses might range from resistant, over tolerant to susceptible. The clinical outcome of infection is highly dependent on the amphibian host, the fungal virulence and environmental determinants. B. dendrobatidis infects the skin of a large range of anurans, urodeles and caecilians, whereas to date the host range of B. salamandrivorans seems limited to urodeles. So far, the epidemic of B. dendrobatidis is mainly limited to Australian, neotropical, South European and West American amphibians, while for B. salamandrivorans it is limited to European salamanders. Other striking differences between both fungi include gross pathology and thermal preferences. With this review we aim to provide the reader with a state-of-the art of host-pathogen interactions for both fungi, in which new data pertaining to the interaction of B. dendrobatidis and B. salamandrivorans with the host's skin are integrated. Furthermore, we pinpoint areas in which more detailed studies are necessary or which have not received the attention they merit.
Figures

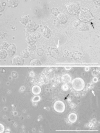
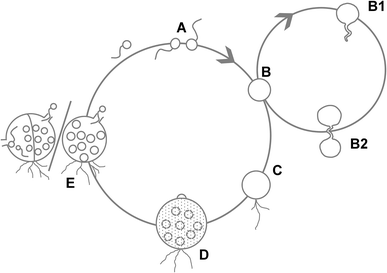


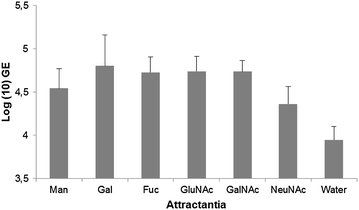
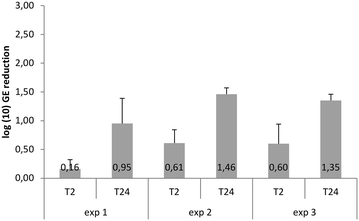

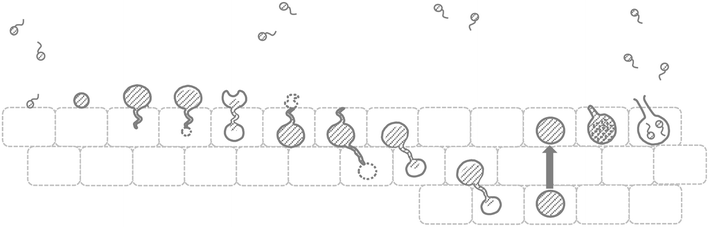
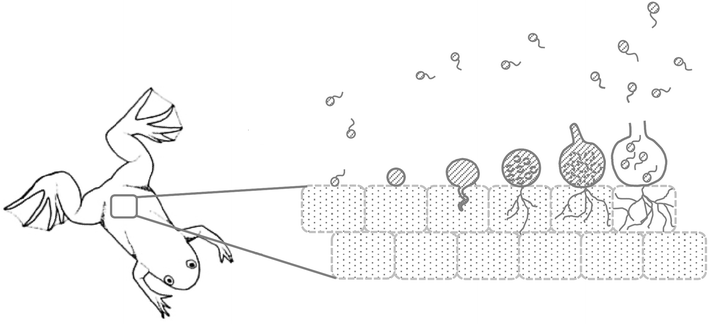
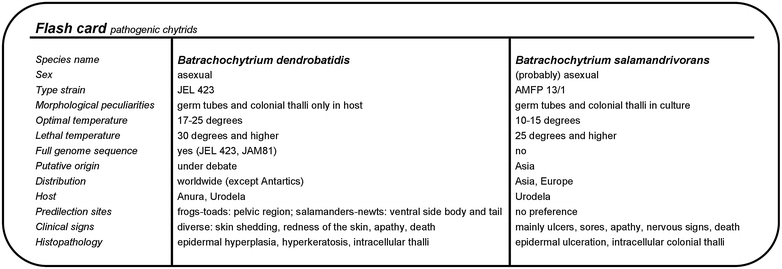
References
-
- IUCN (2015) IUCN Red List of Threatened Species. Version 2015-3. http://www.iucnredlist.org. Accessed 19 Sept 2015
-
- Gascon C, Collins JP, Moore RD, Church DR, McKay JE, Mendelson JR., III . Amphibian conservation action plan. Gland: IUCN/SSC Amphibian Specialist Group; 2007.
-
- Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

