Truncated amelogenin and LRAP transgenes improve Amelx null mouse enamel
- PMID: 26607574
- PMCID: PMC4873476
- DOI: 10.1016/j.matbio.2015.11.005
Truncated amelogenin and LRAP transgenes improve Amelx null mouse enamel
Abstract
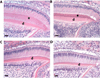
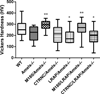
Amelogenin is the most abundant enamel protein involved in enamel mineralization. Our goal was to determine whether all three regions of amelogenin (N-terminus, C-terminus, central core) are required for enamel formation. Amelogenin RNA is alternatively spliced, resulting in at least 16 different amelogenin isoforms in mice, with M180 and LRAP expressed most abundantly. Soon after secretion by ameloblasts, M180 is cleaved by MMP20 resulting in C-terminal truncated (CTRNC) amelogenin. We aimed to determine whether the 2 transgenes (Tg), LRAP and CTRNC together, can improve LRAPTg/Amelx-/- and CTRNCTg/Amelx-/- enamel thickness and prism organization, which were not rescued in Amelx-/- enamel. We generated CTRNCTg/LRAPTg/Amelx-/- mice and analyzed developing and mature incisor and molar enamel histologically, by microCT, SEM and microhardness testing. CTRNCTg and LRAPTg overexpression together significantly improved the enamel phenotype of LRAPTg/Amelx-/- and CTRNCTg/Amelx-/- mouse enamel, however enamel microhardness was recovered only when M180Tg was expressed, alone or with LRAPTg. We determined that both LRAP and CTRNC, which together express all three regions of the amelogenin protein (N-terminus, C-terminus and hydrophobic core) contribute to the final enamel thickness and prism organization in mice.
Keywords: Ameloblasts; Amelogenin; Enamel; MMP20; Secretory stage.
Copyright © 2016 International Society of Matrix Biology. Published by Elsevier B.V. All rights reserved.
Figures






Similar articles
-
Dose-Dependent Rescue of KO Amelogenin Enamel by Transgenes in Vivo.Front Physiol. 2017 Nov 16;8:932. doi: 10.3389/fphys.2017.00932. eCollection 2017. Front Physiol. 2017. PMID: 29201008 Free PMC article.
-
M180 amelogenin processed by MMP20 is sufficient for decussating murine enamel.J Dent Res. 2013 Dec;92(12):1118-22. doi: 10.1177/0022034513506444. Epub 2013 Sep 26. J Dent Res. 2013. PMID: 24072097 Free PMC article.
-
Rescue of the murine amelogenin null phenotype with two amelogenin transgenes.Eur J Oral Sci. 2011 Dec;119 Suppl 1(Suppl 1):70-4. doi: 10.1111/j.1600-0722.2011.00882.x. Eur J Oral Sci. 2011. PMID: 22243230 Free PMC article.
-
Relationship of phenotype and genotype in X-linked amelogenesis imperfecta.Connect Tissue Res. 2003;44 Suppl 1:72-8. Connect Tissue Res. 2003. PMID: 12952177 Review.
-
The use of animal models to explore amelogenin variants in amelogenesis imperfecta.Cells Tissues Organs. 2005;181(3-4):196-201. doi: 10.1159/000091381. Cells Tissues Organs. 2005. PMID: 16612085 Review.
Cited by
-
Mapping the Tooth Enamel Proteome and Amelogenin Phosphorylation Onto Mineralizing Porcine Tooth Crowns.Front Physiol. 2019 Jul 30;10:925. doi: 10.3389/fphys.2019.00925. eCollection 2019. Front Physiol. 2019. PMID: 31417410 Free PMC article.
-
Amelogenin phosphorylation regulates tooth enamel formation by stabilizing a transient amorphous mineral precursor.J Biol Chem. 2020 Feb 14;295(7):1943-1959. doi: 10.1074/jbc.RA119.010506. Epub 2020 Jan 9. J Biol Chem. 2020. PMID: 31919099 Free PMC article.
-
DENTAL ENAMEL FORMATION AND IMPLICATIONS FOR ORAL HEALTH AND DISEASE.Physiol Rev. 2017 Jul 1;97(3):939-993. doi: 10.1152/physrev.00030.2016. Physiol Rev. 2017. PMID: 28468833 Free PMC article. Review.
-
Mechanisms of Enamel Mineralization Guided by Amelogenin Nanoribbons.J Dent Res. 2021 Dec;100(13):1434-1443. doi: 10.1177/00220345211012925. Epub 2021 May 19. J Dent Res. 2021. PMID: 34009057 Free PMC article. Review.
-
Dose-Dependent Rescue of KO Amelogenin Enamel by Transgenes in Vivo.Front Physiol. 2017 Nov 16;8:932. doi: 10.3389/fphys.2017.00932. eCollection 2017. Front Physiol. 2017. PMID: 29201008 Free PMC article.
References
-
- Ryu OH, Fincham AG, Hu CC, Zhang C, Qian Q, Bartlett JD, et al. Characterization of recombinant pig enamelysin activity and cleavage of recombinant pig and mouse amelogenins. J Dent Res. 1999;78:743–750. - PubMed
-
- Brookes SJ, Robinson C, Kirkham J, Bonass WA. Biochemistry and molecular biology of amelogenin proteins of developing dental enamel. Arch Oral Biol. 1995;40:1–14. - PubMed
-
- Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6:84–108. - PubMed
-
- Fincham AG, Moradian-Oldak J. Recent advances in amelogenin biochemistry. Connect Tissue Res. 1995;32:119–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

