Developmental Expression of CYP2B6: A Comprehensive Analysis of mRNA Expression, Protein Content and Bupropion Hydroxylase Activity and the Impact of Genetic Variation
- PMID: 26608082
- PMCID: PMC4931886
- DOI: 10.1124/dmd.115.067546
Developmental Expression of CYP2B6: A Comprehensive Analysis of mRNA Expression, Protein Content and Bupropion Hydroxylase Activity and the Impact of Genetic Variation
Abstract
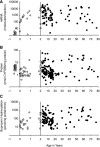
Although CYP2B6 catalyzes the biotransformation of many drugs used clinically for children and adults, information regarding the effects of development on CYP2B6 expression and activity are scarce. Utilizing a large panel of human liver samples (201 donors: 24 fetal, 141 pediatric, and 36 adult), we quantified CYP2B6 mRNA and protein expression levels, characterized CYP2B6 (bupropion hydroxylase) activity in human liver microsomes (HLMs), and performed an extensive genotype analysis to differentiate CYP2B6 haplotypes such that the impact of genetic variation on these parameters could be assessed. Fetal livers contained extremely low levels of CYP2B6 mRNA relative to postnatal samples and fetal HLMs did not appear to catalyze bupropion hydroxylation; however, fetal CYP2B6 protein levels were not significantly different from postnatal levels. Considerable interindividual variation in CYP2B6 mRNA expression, protein levels, and activity was observed in postnatal HLMs (mRNA, ∼40,000-fold; protein, ∼300-fold; activity, ∼600-fold). The extremely wide range of interindividual variability in CYP2B6 expression and activity was significantly associated with age (P < 0.01) following log transformation of the data. Our data suggest that CYP2B6 activity appears as early as the first day of life, increases through infancy, and by 1 year of age, CYP2B6 levels and activity may approach those of adults. Surprisingly, CYP2B6 interindividual variability was not significantly associated with genetic variation in CYP2B6, nor was it associated with differences in gender or ethnicity, suggesting that factors other than these are largely responsible for the wide range of variability in CYP2B6 expression and activity observed among a large group of individuals/samples.
Copyright © 2016 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Al Koudsi N, Tyndale RF. (2010) Hepatic CYP2B6 is altered by genetic, physiologic, and environmental factors but plays little role in nicotine metabolism. Xenobiotica 40:381–392. - PubMed
-
- Bustin SA, Benes V, Garson JA, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. (2009) The MIQE guidelines:minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–22. - PubMed
-
- Court MH, Duan SX, Hesse LM, Venkatakrishnan K, Greenblatt DJ. (2001) Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology 94:110–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

