Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing
- PMID: 26608460
- PMCID: PMC5284479
- DOI: 10.1136/gutjnl-2015-310148
Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing
Abstract
Objective: Gluten-free diet (GFD) is the only management for coeliac disease (CD). Available methods to assess GFD compliance are insufficiently sensitive to detect occasional dietary transgressions that may cause gut mucosal damage. We aimed to develop a method to determine gluten intake and monitor GFD compliance in patients with CD and to evaluate its correlation with mucosal damage.
Design: Urine samples of 76 healthy subjects and 58 patients with CD subjected to different gluten dietary conditions were collected. A lateral flow test (LFT) with the highly sensitive and specific G12 monoclonal antibody for the most dominant gluten immunogenic peptides (GIP) and a LFT reader were used to quantify GIP in solid-phase extracted urines.
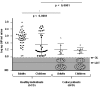
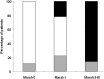
Results: GIP were detectable in concentrated urines from healthy individuals previously subjected to GFD as early as 4-6 h after single gluten intake, and remained detectable for 1-2 days. The urine assay revealed infringement of the GFD in about 50% of the patients. Analysis of duodenal biopsies revealed that most of patients with CD (89%) with no villous atrophy had no detectable GIP in urine, while all patients with quantifiable GIP in urine showed incomplete intestinal mucosa recovery.
Conclusion: GIP are detected in urine after gluten consumption, enabling a new and non-invasive method to monitor GFD compliance and transgressions. The method was sensitive, specific and simple enough to be convenient for clinical monitoring of patients with CD as well as for basic and clinical research applications including drug development.
Trial registration number: NCT02344758.
Keywords: CELIAC DISEASE; GLUTEN FREE DIET.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: ÁC and FL own stock in Biomedal SL. Other authors have declared no conflict of interest. The method of this manuscript was included in a patent application by MLM, CS, AR-H and ÁC as inventors with the assigned number P201400569.
Figures


References
-
- Barratt SM, Leeds JS, Sanders DS. Quality of life in coeliac disease is determined by perceived degree of difficulty adhering to a gluten-free diet, not the level of dietary adherence ultimately achieved. J Gastrointest Liver Dis 2011;20:241–5. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
