Scutellarin regulates microglia-mediated TNC1 astrocytic reaction and astrogliosis in cerebral ischemia in the adult rats
- PMID: 26608466
- PMCID: PMC4660684
- DOI: 10.1186/s12868-015-0219-6
Scutellarin regulates microglia-mediated TNC1 astrocytic reaction and astrogliosis in cerebral ischemia in the adult rats
Abstract
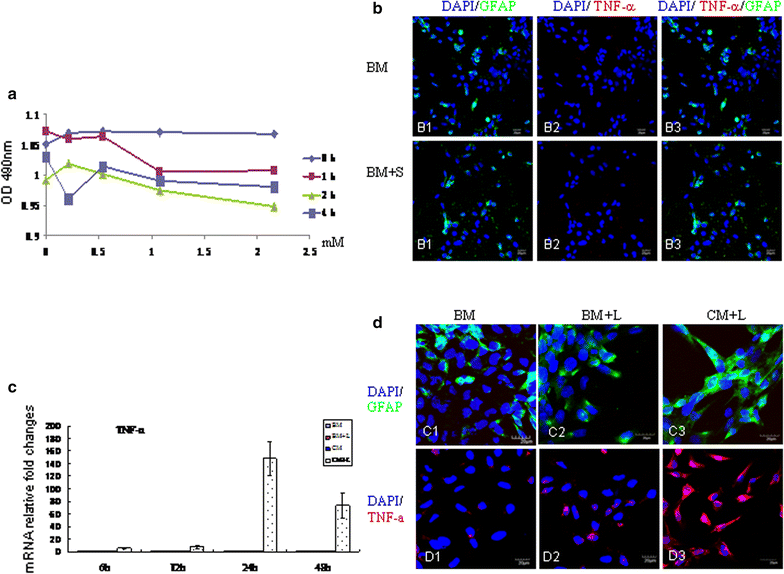
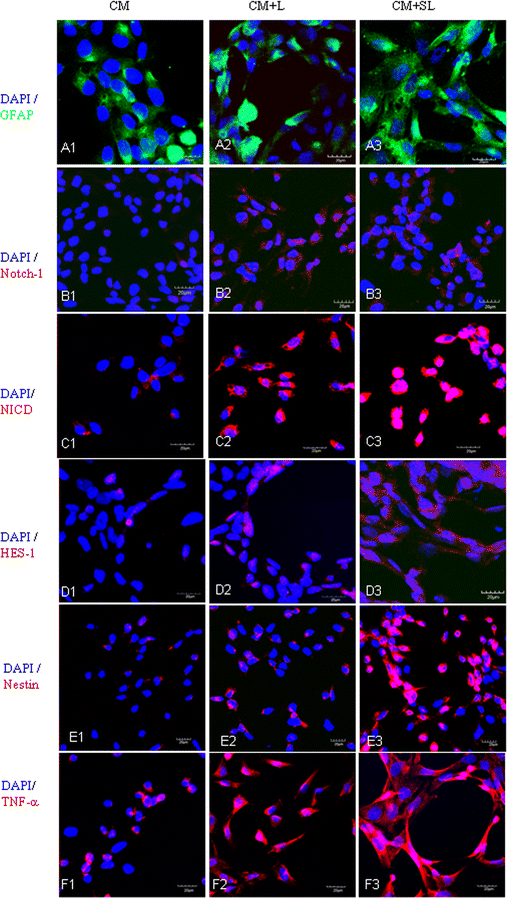
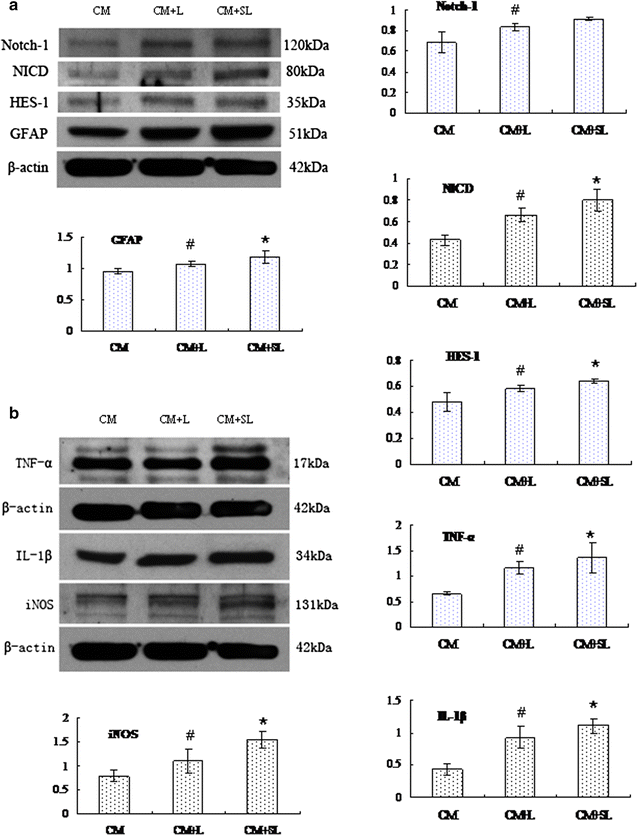
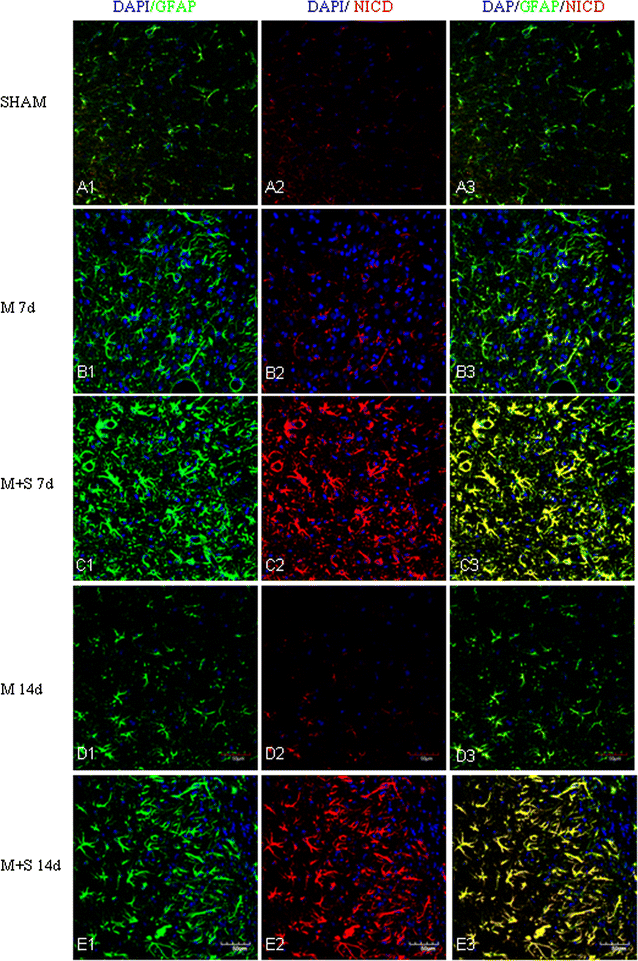
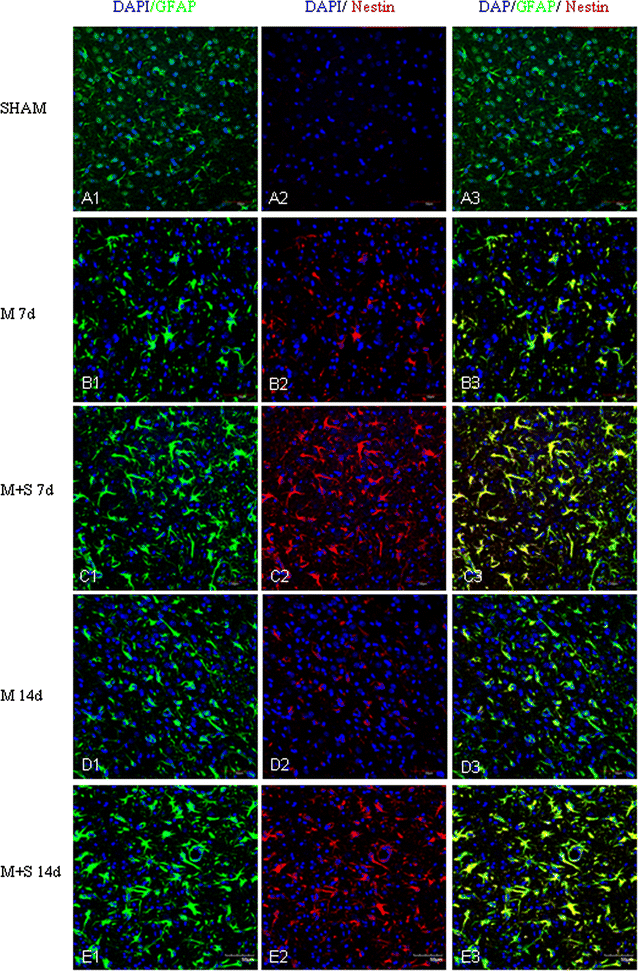
Background: Scutellarin, an anti-inflammatory agent, effectively suppressed microglia activation in rats with middle cerebral artery occlusion (MCAO). Robust microglia activation, acute in onset, was followed by astrogliosis. This study was aimed to determine if scutellarin would also affect the reactive astrocytes that play an important role in tissue repair. Expression of GFAP and Notch-1 and its members: Notch receptor intracellular domain (NICD), and transcription factor hairy and enhancer of split-1 (HES-1), together with nestin and proinflammatory mediators was assessed by immunofluorescence staining in TNC1 astrocytes treated, respectively, with BV-2 conditioned medium (CM) and CM + lipopolysaccharide (LPS) (CM + L) serving as the controls, and conditioned medium derived from LPS-activated BV-2 cells pretreated with scutellarin (CM + SL). Study of the above biomarkers was then extended to reactive astrocytes in scutellarin injected MCAO rats.
Results: TNC1 astrocytes remained relatively unreactive in terms of expression of different biomarkers to direct scutellarin treatment when compared with the control cells. In comparison to cells in the control medium (CM, CM + L), they responded vigorously to CM + SL as evidenced by the enhanced protein expression of GFAP, Notch-1, NICD and HES-1 coupled with that of nestin, TNF-α, IL-1β, and iNOS by Western and immunofluorescence analysis. Electron microscopy showed marked hypertrophy and cell expansion of TNC1 astrocytes bearing many filamentous processes indicative of enhanced astrocyte reaction when treated with CM + SL. In MCAO rats, scutellarin also augmented the expression of the above markers in reactive astrocytes; moreover, astrocytes were evidently hypertrophic.
Conclusions: The results suggest that scutellarin regulates astrogliosis; more importantly, it is microglia-mediated as demonstrated in vitro. Increased expression of Notch signaling in synchrony with nestin may be linked to proliferation and "de-differentiation" of reactive astrocytes; the significance of enhanced TNF-α, IL-1β and iNOS expression in reactive astrocytes by scutellarin may be neuroprotective but this remains speculative.
Figures





Similar articles
-
Scutellarin promotes microglia-mediated astrogliosis coupled with improved behavioral function in cerebral ischemia.Neurochem Int. 2016 Jul;97:154-71. doi: 10.1016/j.neuint.2016.04.007. Epub 2016 Apr 20. Neurochem Int. 2016. PMID: 27105682
-
Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia.BMC Neurosci. 2014 Nov 22;15:125. doi: 10.1186/s12868-014-0125-3. BMC Neurosci. 2014. PMID: 25416145 Free PMC article.
-
Scutellarin regulates the Notch pathway and affects the migration and morphological transformation of activated microglia in experimentally induced cerebral ischemia in rats and in activated BV-2 microglia.J Neuroinflammation. 2015 Jan 20;12:11. doi: 10.1186/s12974-014-0226-z. J Neuroinflammation. 2015. PMID: 25600517 Free PMC article.
-
Scutellarin Attenuates Microglia-Mediated Neuroinflammation and Promotes Astrogliosis in Cerebral Ischemia - A Therapeutic Consideration.Curr Med Chem. 2017;24(7):718-727. doi: 10.2174/0929867324666161118142045. Curr Med Chem. 2017. PMID: 27855618 Review.
-
Scutellarin as a Potential Therapeutic Agent for Microglia-Mediated Neuroinflammation in Cerebral Ischemia.Neuromolecular Med. 2016 Sep;18(3):264-73. doi: 10.1007/s12017-016-8394-x. Epub 2016 Apr 21. Neuromolecular Med. 2016. PMID: 27103430 Review.
Cited by
-
Combined Scutellarin and C18H17NO6 Imperils the Survival of Glioma: Partly Associated With the Repression of PSEN1/PI3K-AKT Signaling Axis.Front Oncol. 2021 Sep 9;11:663262. doi: 10.3389/fonc.2021.663262. eCollection 2021. Front Oncol. 2021. PMID: 34568005 Free PMC article.
-
Inflammatory factors and amyloid β-induced microglial polarization promote inflammatory crosstalk with astrocytes.Aging (Albany NY). 2020 Nov 16;12(22):22538-22549. doi: 10.18632/aging.103663. Epub 2020 Nov 16. Aging (Albany NY). 2020. PMID: 33196457 Free PMC article.
-
Scutellarin ameliorates neonatal hypoxic-ischemic encephalopathy associated with GAP43-dependent signaling pathway.Chin Med. 2021 Oct 18;16(1):105. doi: 10.1186/s13020-021-00517-z. Chin Med. 2021. PMID: 34663387 Free PMC article.
-
Induction of Ischemic Stroke and Ischemia-reperfusion in Mice Using the Middle Artery Occlusion Technique and Visualization of Infarct Area.J Vis Exp. 2017 Feb 2;(120):54805. doi: 10.3791/54805. J Vis Exp. 2017. PMID: 28190061 Free PMC article.
-
Neuroprotective Phytochemicals in Experimental Ischemic Stroke: Mechanisms and Potential Clinical Applications.Oxid Med Cell Longev. 2021 Apr 28;2021:6687386. doi: 10.1155/2021/6687386. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34007405 Free PMC article. Review.
References
-
- Liu H, Yang X, Tang R, Liu J, Xu H. Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol Res Off J Ital Pharmacol Soc. 2005;51(3):205–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

