Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening
- PMID: 26608672
- PMCID: PMC4660439
- DOI: 10.1038/srep17189
Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening
Abstract
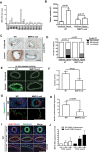
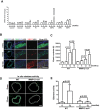
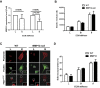
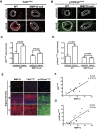
Arterial stiffening is a hallmark of aging and risk factor for cardiovascular disease, yet its regulation is poorly understood. Here we use mouse modeling to show that matrix metalloproteinase-12 (MMP12), a potent elastase, is essential for acute and chronic arterial stiffening. MMP12 was induced in arterial smooth muscle cells (SMCs) after acute vascular injury. As determined by genome-wide analysis, the magnitude of its gene induction exceeded that of all other MMPs as well as those of the fibrillar collagens and lysyl oxidases, other common regulators of tissue stiffness. A preferential induction of SMC MMP12, without comparable effect on collagen abundance or structure, was also seen during chronic arterial stiffening with age. In both settings, deletion of MMP12 reduced elastin degradation and blocked arterial stiffening as assessed by atomic force microscopy and immunostaining for stiffness-regulated molecular markers. Isolated MMP12-null SMCs sense extracellular stiffness normally, indicating that MMP12 causes arterial stiffening by remodeling the SMC microenvironment rather than affecting the mechanoresponsiveness of the cells themselves. In human aortic samples, MMP12 levels strongly correlate with markers of SMC stiffness. We conclude that MMP12 causes arterial stiffening in mice and suggest that it functions similarly in humans.
Figures
References
-
- Duprez D. A. & Cohn J. N. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr. Atheroscler. Rep. 9, 139–144 (2007). - PubMed
-
- Sutton-Tyrrell K. et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 111, 3384–3390 (2005). - PubMed
-
- Van Popele N. M. et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 32, 454–460 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

