Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction
- PMID: 26608695
- PMCID: PMC10559340
- DOI: 10.1002/14651858.CD009749.pub2
Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction
Update in
-
Androgens (dehydroepiandrosterone or testosterone) for women undergoing assisted reproduction.Cochrane Database Syst Rev. 2024 Jun 5;6(6):CD009749. doi: 10.1002/14651858.CD009749.pub3. Cochrane Database Syst Rev. 2024. PMID: 38837771 Free PMC article.
Abstract
Background: Infertility is a condition affecting 10% to 15% of couples of reproductive age. It is generally defined as "the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse". The treatment of infertility may involve manipulation of gametes or of the embryos themselves. These techniques are together known as assisted reproductive technology (ART). Practitioners are constantly seeking alternative or adjunct treatments, or both, in the hope that they may improve the outcome of assisted reproductive techniques. This Cochrane review focusses on the adjunct use of synthetic versions of two naturally-produced hormones, dehydroepiandrosterone (DHEA) and testosterone (T), in assisted reproduction.DHEA and its derivative testosterone are steroid hormones proposed to increase conception rates by positively affecting follicular response to gonadotrophin stimulation, leading to greater oocyte yields and, in turn, increased chance of pregnancy.
Objectives: To assess the effectiveness and safety of DHEA and testosterone as pre- or co-treatments in subfertile women undergoing assisted reproduction.
Search methods: We searched the following electronic databases, trial registers and websites up to 12 March 2015: the Cochrane Central Register of Controlled Trials (CENTRAL), the Menstrual Disorders and Subfertility Group (MDSG) Specialised Register, MEDLINE, EMBASE, PsycINFO, CINAHL, electronic trial registers for ongoing and registered trials, citation indexes, conference abstracts in the Web of Science, PubMed and OpenSIGLE. We also carried out handsearches. There were no language restrictions.
Selection criteria: We included randomised controlled trials (RCTs) comparing DHEA or testosterone as an adjunct treatment to any other active intervention, placebo, or no treatment in women undergoing assisted reproduction.
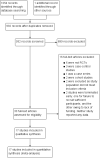
Data collection and analysis: Two review authors independently selected studies, extracted relevant data and assessed them for risk of bias. We pooled studies using fixed-effect models. We calculated odds ratios (ORs) for each dichotomous outcome. Analyses were stratified by type of treatment. There were no data for the intended groupings by dose, mode of delivery or after one/more than one cycle.We assessed the overall quality of the evidence for the main findings using the GRADE working group methods.
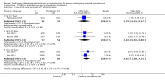
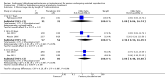
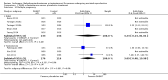
Main results: We included 17 RCTs with a total of 1496 participants. Apart from two trials, the trial participants were women identified as 'poor responders' to standard IVF protocols. The included trials compared either testosterone or DHEA treatment with placebo or no treatment.When DHEA was compared with placebo or no treatment, pre-treatment with DHEA was associated with higher rates of live birth or ongoing pregnancy (OR 1.88, 95% CI 1.30 to 2.71; eight RCTs, N = 878, I² statistic = 27%, moderate quality evidence). This suggests that in women with a 12% chance of live birth/ongoing pregnancy with placebo or no treatment, the live birth/ongoing pregnancy rate in women using DHEA will be between 15% and 26%. However, in a sensitivity analysis removing trials at high risk of performance bias, the effect size was reduced and no longer reached significance (OR 1.50, 95% CI 0.88 to 2.56; five RCTs, N = 306, I² statistic = 43%). There was no evidence of a difference in miscarriage rates (OR 0.58, 95% CI 0.29 to 1.17; eight RCTs, N = 950, I² statistic = 0%, moderate quality evidence). Multiple pregnancy data were available for five trials, with one multiple pregnancy in the DHEA group of one trial (OR 3.23, 95% CI 0.13 to 81.01; five RCTs, N = 267, very low quality evidence).When testosterone was compared with placebo or no treatment we found that pre-treatment with testosterone was associated with higher live birth rates (OR 2.60, 95% CI 1.30 to 5.20; four RCTs, N = 345, I² statistic = 0%, moderate evidence). This suggests that in women with an 8% chance of live birth with placebo or no treatment, the live birth rate in women using testosterone will be between 10% and 32%. On removal of studies at high risk of performance bias in a sensitivity analysis, the remaining study showed no evidence of a difference between the groups (OR 2.00, 95% CI 0.17 to 23.49; one RCT, N = 53). There was no evidence of a difference in miscarriage rates (OR 2.04, 95% CI 0.58 to 7.13; four RCTs, N = 345, I² = 0%, low quality evidence). Multiple pregnancy data were available for three trials, with four events in the testosterone group and one in the placebo/no treatment group (OR 3.09, 95% CI 0.48 to 19.98; three RCTs, N = 292, very low quality evidence).One study compared testosterone with estradiol and reported no evidence of a difference in live birth rates (OR 2.06, 95% CI 0.43 to 9.87; one RCT, N = 46, very low quality evidence) or miscarriage rates (OR 0.70, 95% CI 0.11 to 4.64; one RCT, N = 46, very low quality evidence).The quality of the evidence was moderate, the main limitations being lack of blinding in the included trials, inadequate reporting of study methods, and low event and sample sizes in some trials.
Authors' conclusions: In women identified as poor responders undergoing ART, pre-treatment with DHEA or testosterone may be associated with improved live birth rates. The overall quality of the evidence is moderate. There is insufficient evidence to draw any conclusions about the safety of either androgen. Definitive conclusions regarding the clinical role of either androgen awaits evidence from further well-designed studies.
Conflict of interest statement
None known
Figures
















References
References to studies included in this review
Artini 2012 {published data only}
-
- Artini PG, Simi G, Ruggiero M, Pinelli S, Berardino OM, Papini F, et al. DHEA supplementation improves follicular microenvironment in poor responder patients. Gynecological Endocrinology 2012;28(9):669–73. - PubMed
Divita 2003 {published data only}
-
- Divita AE, Kanzepolsky LS, Notrica JA, Neuspiller FD, Polak de Fried E. Does dehidroepiandrosterone sulfate (Dheas) co‐treatment improve art outcome? A prospective, randomized, double‐blind placebo‐controlled trial. Fertility and Sterility 2003;80 Suppl 3:S111‐2.
Evans 2013 {published data only}
-
- Evans J. A randomised, double blinded, placebo controlled, single centre trial comparing dehydroepiandrosterone (DHEA) and placebo in the treatment of women with resistant ovaries prior to in vitro fertilisation (IVF) and intra cytoplasmic sperm injection (ICSI). RCOG World Congress; 2013 June 24‐26; Liverpool, UK. BJOG. 2013; Vol. EP2.93:225.
Fábregues 2009 {published data only}
-
- Fábregues F, Peñarrubia J, Creus M, Manau D, Casals G, Carmona F, et al. Transdermal testosterone may improve ovarian response to gonadotrophins in low‐responder IVF patients: a randomized, clinical trial. Human Reproduction 2009;24(2):349‐59. - PubMed
Jindal 2014 {published and unpublished data}
-
- Jindal A, Singh R. A prospective randomised controlled study on the role of dehydroepiandrosterone (DHEA) on improving ovarian response in known poor responders in previous failed IVF‐ICSI cycles. Human Reproduction. 2014; Vol. 29:Supp 1, i14.
Kara 2014 {published data only}
-
- Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Does dehydroepiandrosterone supplementation really affect IVF‐ICSI outcome in women with poor ovarian reserve?. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2014;173:63‐5. - PubMed
-
- Kara M, Aydin T, Aran T, Turktekin N, Ozdemir B. Is dehydroepiandrosterone (DHEA) supplementation really effective on IVF‐ICSI outcome in patients with poor ovarian reserve?. Fertility and Sterility 2013;100(3, Supplement):S518.
Kim 2010 {published and unpublished data}
-
- Kim CH, Ahn JW, Nah HY, Kim SH, Chae HD, Kang BM. Ovarian features after 2 weeks, 3 weeks and 4 weeks transdermal testosterone gel treatment and their associated effect on IVF/ICSI outcome in low responders. Fertility and Sterility 2010;94(4 Suppl):S155‐6.
Kim 2011 {published data only}
-
- Kim CH, Howles CM, Lee HA. The effect of transdermal testosterone gel pretreatment on controlled ovarian stimulation and IVF outcome in low responders. Fertility and Sterility 2011;95(2):679‐83. - PubMed
Marzal 2014 {unpublished data only}
-
- Díaz C, Pellicer A, Marzal A. Antral Follicle Priming Prior to ICSI (Intracytoplasmic Sperm Injection) in Previously Diagnosed Low Responders (FOLLPRIM). https://clinicaltrials.gov/ct2/show/NCT01310647 (accessed 21 May 2014). [NCT01310647]
-
- Díaz C, Pellicer A, Marzal A. Antral Follicle Priming Prior to ICSI (Intracytoplasmic Sperm Injection) in Previously Diagnosed Low Responders (FOLLPRIM). personal correspondence 2014.
-
- Marzal A, Monterde M, Díaz‐García C, Gómez R, Rubio JM. Antral follicle priming prior to ICSI in confirmed low responders: a randomized clinical trial (FOLLPRIM). Human Reproduction 2014;29(Supp 1):i25.
Massin 2006 {published data only}
-
- Massin N, Cedrin‐Durnerin I, Coussieu C, Galey‐Fontaine J, Wolf JP, Hugues JN. Effects of transdermal testosterone application on the ovarian response to FSH in poor responders undergoing assisted reproduction technique‐‐a prospective, randomized, double‐blind study. Human Reproduction 2006;21(5):1204‐11. - PubMed
Moawad 2012 {published data only}
-
- Moawad M, Shaeer M. Long‐term androgen priming by use of dehydroepiandrosterone (DHEA) improves IVF outcome in poor‐responder patients. A randomized controlled study. Middle East Fertility Society Journal 2012;17(4):268–74.
Tartagni 2015a {published data only}
-
- Tartagni M, Pergola G, Damiani GR, Pellegrino A, Baldini D, Tartagni MV, et al. Potential benefit of dehydroepiandrosterone supplementation for infertile but not poor responder patients in a IVF program. Minerva Ginecologica 2015;67(1):7‐12. - PubMed
Tartagni 2015b {published data only}
-
- Tartagni M, Cicinelli MV, Baldini D, Tartagni MV, Alrasheed H, DeSalvia MA, et al. Dehydroepiandrosterone decreases the age‐related decline of the in vitro fertilization outcome in women younger than 40 years old. Reproductive Biology and Endocrinology 2015;13:13‐8. [DOI: 10.1186/s12958-015-0014-3] - DOI - PMC - PubMed
Wiser 2010 {published data only}
-
- Wiser A, Gonen O, Ghetler Y, Shavit T, Berkovitz A, Shulman A. Addition of dehydroepiandrosterone (DHEA) for poor‐responder patients before and during IVF treatment improves the pregnancy rate: a randomized prospective study. Human Reproduction 2010;25(10):2496‐500. - PubMed
Yeung 2013a {published data only}
-
- Yeung TWY, Li RHW, Lee VCY, Chai J, Ng EHY, Ho PC. A randomized double‐blinded placebo‐controlled trial on the effect of 16 weeks of dehydroepiandrosterone (DHEA) on ovarian reserve markers and IVF outcomes in normal responders. Fertility and Sterility 2013;100(3, Supplement):S114.
Yeung 2014 {published data only}
-
- Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. A randomized double‐blinded placebo‐controlled trial on the effect of 16 weeks of dehydroepiandrosterone (DHEA) on ovarian reserve markers and IVF outcomes in poor responders. Fertility and Sterility 2013;100(3, Suppl):S159‐60.
-
- Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. A randomized, controlled, pilot trial on the effect of dehydroepiandrosterone on ovarian response markers, ovarian response, and in vitro fertilization outcomes in poor responders. Fertility and Sterility 2014;102(1):108‐15.e1. [DOI: 10.1016/j.fertnstert.2014.03.044] - DOI - PubMed
Zhang 2014 {published data only}
-
- Zhang HH, Xu PY, Wu J, Zou WW, Xu XM, Cao XY, et al. Dehydroepiandrosterone improves follicular fluid bone morphogenetic protein‐15 and accumulated embryo score of infertility patients with diminished ovarian reserve undergoing in vitro fertilization: a randomized controlled trial. Journal of Ovarian Research 2014;7:93. - PMC - PubMed
References to studies excluded from this review
Balasch 2006 {published data only}
-
- Balasch J, Fábregues F, Peñarrubia J, Carmona F, Casamitjana R, Creus M, et al. Pretreatment with transdermal testosterone may improve ovarian response to gonadotrophins in poor‐responder IVF patients with normal basal concentrations of FSH. Human Reproduction 2006;21(7):1884‐93. - PubMed
Barad 2006 {published data only}
-
- Barad D, Gleicher N. Effect of dehydroepiandrosterone on oocyte and embryo yields, embryo grade and cell number in IVF. Human Reproduction 2006;21(11):2845‐9. - PubMed
Barad 2007 {published data only}
Barad 2008b {unpublished data only}
-
- Barad D. A Trial of Dehydroepiandrosterone (DHEA) Treatment for in Vitro Fertilization (IVF). https://clinicaltrials.gov/ct2/show/NCT00419913 (accessed 12 March 2015). [NCT00419913]
Casson 2000 {published data only}
-
- Casson PR, Lindsay MS, Pisarska MD, Carson SA, Buster JE. Dehydroepiandrosterone supplementation augments ovarian stimulation in poor responders: a case series. Human Reproduction 2000;15(10):2129‐32. - PubMed
de los Santos 2013 {published data only}
-
- Santos MJ, García‐Laez V, Beltrán D, Labarta E, Zuzuarregui JL, Alamá P, et al. The follicular hormonal profile in low‐responder patients undergoing unstimulated cycles: is it hypoandrogenic?. Human Reproduction 2013;28(1):224‐9. - PubMed
Fábregues 2011 {unpublished data only}
-
- Fábregues F. Efficacy of Luteinizing Hormone (LH) Activity in Low Responder Patients With Transdermal Testosterone. https://clinicaltrials.gov/ct2/show/NCT01291212 (accessed 12 March 2015). [NCT01291212]
Fusi 2013 {published data only}
-
- Fusi FM, Ferrario M, Bosisio C, Arnoldi M, Zanga L. DHEA supplementation positively affects spontaneous pregnancies in women with diminished ovarian function. Gynecological Endocrinology 2013;29(10):940–3. - PubMed
Gleicher 2013 {published data only}
-
- Gleicher N, Kim A, Weghofer A, Shohat‐Ta A, Lazzaroni E, Lee HJ, et al. Starting and resulting testosterone levels after androgen supplementation determine at all ages in vitro fertilization (IVF) pregnancy rates in women with diminished ovarian reserve (DOR). Journal of Assisted Reproduction and Genetics 2013;30(1):49‐62. - PMC - PubMed
Hyman 2013 {published data only}
-
- Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Algur N, Eldar‐Geva T. Dehydroepiandrosterone (DHEA) supplementation for poor responders ‐ how does it work?. Fertility and Sterility 2010;94(4, Supplement):S86.
-
- Hyman JH, Margalioth EJ, Rabinowitz R, Tsafrir A, Gal M, Alerhand S, et al. DHEA supplementation may improve IVF outcome in poor responders: a proposed mechanism. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2013;168(1):49–53. - PubMed
Monterde 2013 {published data only}
-
- Monterde M, Gomez R, Marzal A, Vega O, Rubio J, Diaz‐Garcia C, et al. Molecular expression of androgen and FSH receptors after follicular priming prior to ICSI cycles ‐ an interim analysis of the IFILL‐PRIMI randomised controlled trial. Human Reproduction 2013;28(Suppl 1):i342.
Motta 2006 {published data only}
-
- Motta EL, Rossi LM, Fernandes TR, Massaguer AA, Fassolas G, Serafini P. The use of DHEA in poor responders does not improve IVF outcomes: insights of a pilot study. Fertility and Sterility 2006;86(Suppl 2):S428.
Singh 2013 {published data only}
-
- Singh N, Zangmo R, Kumar S, Roy KK, Sharma JB, Malhotra N, et al. A prospective study on role of dehydroepiandrosterone (DHEA) on improving the ovarian reserve markers in infertile patients with poor ovarian reserve. Gynecological Endocrinology 2013;29(11):989–92. - PubMed
Sipe 2010 {published data only}
-
- Sipe CS, Thomas MR, Stegmann BJ, Voorhis BJ. Effects of exogenous testosterone supplementation in gonadotrophin stimulated cycles. Human Reproduction 2010;25(3):690‐6. - PubMed
Sönmezer 2009 {published data only}
-
- Sönmezer M, Ozmen B, Çil AP, Ozkavukçu S, Taşçi T, Olmuş H, et al. Dehydroepiandrosterone supplementation improves ovarian response and cycle outcome in poor responders. Reproductive BioMedicine Online 2009;19(4):508‐13. - PubMed
Yeung 2013b {published data only}
-
- Yeung TW, Li RH, Lee VC, Ho PC, Ng EH. A randomized double‐blinded placebo‐controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. Journal of Clinical Endocrinology and Metabolism 2013;98(1):380–8. - PubMed
References to ongoing studies
Barad 2008a {unpublished data only}
-
- Study of Oral Dehydroepiandrosterone (DHEA) to Treat Previously Unexplained Infertility. Ongoing study March 2008.
Barad 2012 {unpublished data only}
-
- A Randomized Double Blind Control Trial of Transdermal Testosterone Supplementation vs Placebo on Follicular Development and Atresia, Oocyte and Embryo Quality Among Women With Diminished Ovarian Reserve Undergoing in Vitro Fertilization. Ongoing study July 2012End date: December 2014.
Jayaprakasan 2012 {published data only}
Kolibianakis 2014 {unpublished data only}
-
- Transdermal Testosterone Pretreatment in Poor Responders Undergoing IVF. Ongoing study October 2013Estimated primary completion date: October 2014 (final data collection date for primary outcome measure).
Tsafrir 2007 {unpublished data only}
-
- DHEA Supplementation for Low Ovarian Response IVF Patients. Ongoing study Jan 2008.
Viardot‐Foucault 2012 {unpublished data only}
-
- Study of Dehydroepiandrosterone Treatment for Poor Responders in In Vitro Fertilization Patients. Ongoing study February 2012.
Vlahos 2014 {unpublished data only}
-
- Prospective Randomized Trial on the Effect of DHEA Administration in Women With Poor Ovarian Reserve Undergoing Controlled Ovarian Stimulation for IVF. Impact on Stimulation Characteristics and Pregnancy Outcome.. Ongoing study April 2014.
Additional references
Akhtar 2013
Bosdou 2012
-
- Bosdou JK, Venetis CA, Kolibianakis EM, Toulis KA, Goulis DG, Zepiridis l, et al. The use of androgens or androgen‐modulating agents in poor responders undergoing in vitro fertilization: a systematic review and meta‐analysis. Human Reproduction Update 2012;18(2):127‐45. - PubMed
Buvat 2003
-
- Buvat J. Androgen therapy with dehydroepiandrosterone. World Journal of Urology 2003;21(5):346‐55. - PubMed
Casson 1998
-
- Casson PR, Santoro N, Elkind‐Hirsch K, Carson SA, Hornsby PJ, Abraham G, et al. Postmenopausal dehydroepiandrosterone administration increases free insulin‐like growth factor‐I and decreases high‐density lipoprotein: a six‐month trial. Fertility and Sterility 1998;70(1):107‐10. - PubMed
Cheong 2013
CONSORT 2010
-
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Journal of Clinical Epidemiology 2010;63(8):834‐40. - PubMed
Davison 2005
-
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. Journal of Clincal Endocrinology and Metabolism 2005;90(7):3847‐53. - PubMed
Fouany 2013
Frattarelli 2004
-
- Frattarelli JL, Peterson EH. Effect of androgen levels on in vitro fertilization cycles. Fertility and Sterility 2004;81(6):1713‐4. - PubMed
Garcia‐Velasco 2005
-
- Garcia‐Velasco JA, Moreno L, Pacheco A, Guillén A, Duque L, Requena A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertility and Sterility 2005;84(1):82‐7. - PubMed
Genazzani 2001
-
- Genazzani AD, Stomati M, Strucchi C, Puccetti S, Luisi S, Genazzani AR. Oral dehydroepiandrosterone supplementation modulates spontaneous and growth hormone‐releasing hormone‐induced growth hormone and insulin‐like growth factor‐1 secretion in early and late postmenopausal women. Fertility and Sterility 2001;76(2):241‐8. - PubMed
Gleicher 2009
Gleicher 2011a
-
- Gleicher N, Barad D. Dehydroepiandrosterone. In: Kovacs G editor(s). How to improve your ART success rates. Cambridge: Cambridge University Press, 2011:93‐8.
Gleicher 2011b
Gnoth 2005
-
- Gnoth C, Godehardt E, Frank‐Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144‐7. - PubMed
González‐Comadran 2012
-
- González‐Comadran M, Durán M, Solà I, Fábregues F, Carreras R, Checa MA. Effects of transdermal testosterone in poor responders undergoing IVF: systematic review and meta‐analysis. Reproductive BioMedicine Online 2012;25(5):450–9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horie 1992
-
- Horie K, Takakura K, Imai K, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Human Reproduction 1992;7(10):1461‐6. - PubMed
Karp 2009
-
- Karp G, Bentov Y, Masalha R, Ifergane G. Onset of late posttraumatic seizure after dehydroepiandrosterone treatment. Fertility and Sterility 2009;91(3):931.e1‐2. - PubMed
Mamas 2009a
-
- Mamas L, Mamas E. Premature ovarian failure and dehydroepiandrosterone. Fertility and Sterility 2009;91(2):644‐6. - PubMed
Mamas 2009b
-
- Mamas L, Mamas E. Dehydroepiandrosterone supplementation in assisted reproduction: rationale and results. Current Opinion in Obstetrics and Gynaecology 2009;21(4):306‐8. - PubMed
Morales 1994
-
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. Journal of Clinical Endocrinology and Metabolism 1994;78(6):1360‐7. - PubMed
Nielsen 2011
-
- Nielsen ME, Rasmussen IA, Kristensen SG, Christensen ST, Møllgård K, Wreford Andersen E, et al. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Molecular Human Reproduction 2011;17(1):63‐70. - PubMed
Review Manager (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 1968
-
- Ryan KJ, Petro Z, Kaiser J. Steroid formation by isolated and recombined ovarian granulosa and thecal cells. Journal of Clinical Endocrinology & Metabolism 1968;28(3):355‐8. - PubMed
Sen 2010
Sir‐Petermann 2002
-
- Sir‐Petermann T, Maliqueo M, Angel B, Lara HE, Pérez‐Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction 2002;17(10):2573‐9. - PubMed
Siristatidis 2011
Somboonporn 2005
Sunkara 2011
-
- Sunkara SK, Pundir J, Khalaf Y. Effect of androgen supplementation or modulation on ovarian stimulation outcome in poor responders: a meta‐analysis. Reproductive BioMedicine Online 2011;22(6):545‐55. - PubMed
Toner 2002
-
- Toner JP. Progress we can be proud of: U.S. trends in assisted reproduction over the first 20 years. Fertility and Sterility 2002;78(5):943‐50. - PubMed
Vendola 1999
-
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin‐like growth factor I expression and initiation of follicle development in the primate ovary. Biology of Reproduction 1999;61(2):353–7. - PubMed
Venetis 2011
-
- Venetis CA, Bosdou J, Kolibianakis E, Toulis K, Goulis D, Tarlatzis BC. Improved probability of pregnancy with transdermal testosterone pretreatment in poor responders treated with GnRH analogues and gonadotrophins for in‐vitro fertilization: a meta‐analysis. Fertility and Sterility 2011;96(3):S56.
Weil 1998
-
- Weil SJ, Vendola K, Zhou J, Adesanya OO, Wang J, Okafor J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. Journal of Clinical Endocrinology and Metabolism 1998;83(7):2479‐85. - PubMed
Weil 1999
-
- Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle‐stimulating hormone interactions in primate ovarian follicle development. Journal of Clinical Endocrinology and Metabolism 1999;84(8):2951‐6. - PubMed
Yakin 2011
-
- Yakin K, Urman B. DHEA as a miracle drug in the treatment of poor responders; hype or hope?. Human Reproduction 2011;26(8):1941–4. - PubMed
Zegers‐Hochschild 2009
-
- Zegers‐Hochschild F, Adamson GD, Mouzon J, Ishihara O, Mansour R, Nygren K, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertility and Sterility 2009;92(5):1520‐4. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

