The Tumor Antigen NY-ESO-1 Mediates Direct Recognition of Melanoma Cells by CD4+ T Cells after Intercellular Antigen Transfer
- PMID: 26608910
- PMCID: PMC4683358
- DOI: 10.4049/jimmunol.1402664
The Tumor Antigen NY-ESO-1 Mediates Direct Recognition of Melanoma Cells by CD4+ T Cells after Intercellular Antigen Transfer
Abstract
NY-ESO-1-specific CD4(+) T cells are of interest for immune therapy against tumors, because it has been shown that their transfer into a patient with melanoma resulted in tumor regression. Therefore, we investigated how NY-ESO-1 is processed onto MHC class II molecules for direct CD4(+) T cell recognition of melanoma cells. We could rule out proteasome and autophagy-dependent endogenous Ag processing for MHC class II presentation. In contrast, intercellular Ag transfer, followed by classical MHC class II Ag processing via endocytosis, sensitized neighboring melanoma cells for CD4(+) T cell recognition. However, macroautophagy targeting of NY-ESO-1 enhanced MHC class II presentation. Therefore, both elevated NY-ESO-1 release and macroautophagy targeting could improve melanoma cell recognition by CD4(+) T cells and should be explored during immunotherapy of melanoma.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures
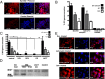
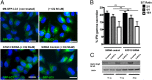
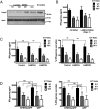


References
-
- Mandic M., Castelli F., Janjic B., Almunia C., Andrade P., Gillet D., Brusic V., Kirkwood J. M., Maillere B., Zarour H. M. 2005. One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J. Immunol. 174: 1751–1759. - PubMed
-
- Gnjatic S., Atanackovic D., Jäger E., Matsuo M., Selvakumar A., Altorki N. K., Maki R. G., Dupont B., Ritter G., Chen Y. T., et al. 2003. Survey of naturally occurring CD4+ T cell responses against NY-ESO-1 in cancer patients: correlation with antibody responses. Proc. Natl. Acad. Sci. USA 100: 8862–8867. - PMC - PubMed
-
- Cho H. J., Caballero O. L., Gnjatic S., Andrade V. C., Colleoni G. W., Vettore A. L., Outtz H. H., Fortunato S., Altorki N., Ferrera C. A., et al. 2006. Physical interaction of two cancer-testis antigens, MAGE-C1 (CT7) and NY-ESO-1 (CT6). Cancer Immun. 6: 12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

