Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation
- PMID: 26609283
- PMCID: PMC4657531
Production, purification and characterization of novel beta glucosidase from newly isolated Penicillium simplicissimum H-11 in submerged fermentation
Abstract
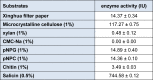
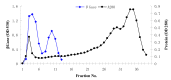
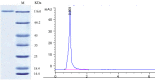
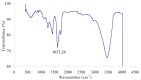
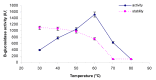
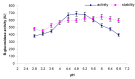
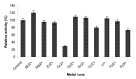
β-Glucosidase is an important component of the cellulase complex. It not only hydrolyzes cellobiose and short-chain cellooligosaccharides to glucose, but also removes the inhibitory effect of cellobiose on the β-1, 4-endoglucanase and exoglucanase, thereby increasing the overall rate of cellulose biodegradation. β-glucosidasefrom culture supernatant of a fungus Penicillium simplicissimum was purified to homogeneity, by using ammonium sulfate fraction, Sephadex G-100 chromatography, and its properties were studied. The molecular mass of the enzyme was about 126.0 kDa, as identified by 12% SDS-PAGE. The optimum pH and temperature were 4.4 ~ 5.2 and 60 °C, respectively. The enzyme was stable in pH 5.2 ~ 6.4 and under 40 °C. Metal profile of the enzyme showed that Mn(2+) enhances its activity, while Cu(2+), Co(2+)and Fe(3+) cause obvious inhibition. The K m and V max was 14.881 mg/ml and 0.364 mg ml/min against salicin as a Substrate. This enzyme had secondary protein structure as evidenced by FTIR spectrum.
Keywords: Penicillium simplicissimum; beta-glucosidase; characterization; purification.
Figures



References
-
- Banker J. Amide modes and protein conformation. Biochim Biophys Acta. 1992;1120:123−43. - PubMed
-
- Begum MF. Bangladesh: University of Rajshahi, Department of Botany; 2005. Screening of Aspergilli from cellulosic waste materials and studies on their cellulolytic properties. Ph.D. thesis.
-
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–83. - PubMed
-
- Bhatia Y, Mishra S, Bisaria VS. Microbial β-glucosidases: cloning, properties, and applications. Crit Rev Biotechnol. 2002;22:375–407. - PubMed
LinkOut - more resources
Full Text Sources
