Beclin-1-independent autophagy mediates programmed cancer cell death through interplays with endoplasmic reticulum and/or mitochondria in colbat chloride-induced hypoxia
- PMID: 26609472
- PMCID: PMC4633894
Beclin-1-independent autophagy mediates programmed cancer cell death through interplays with endoplasmic reticulum and/or mitochondria in colbat chloride-induced hypoxia
Abstract
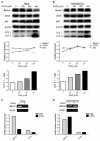
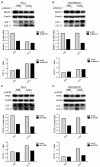
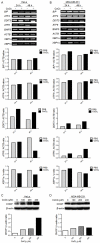
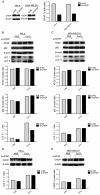
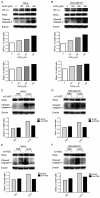
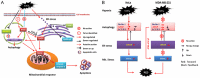
Autophagy has dual functions in cell survival and death. However, the effects of autophagy on cancer cell survival or death remain controversial. In this study, we show that Autophagy can mediate programmed cell death (PCD) of cancer cells in responding to cobalt chloride (CoCl2)-induced hypoxia in a Beclin-1-independent but autophagy protein 5 (ATG5)-dependent manner. Although ATG5 is not directly induced by CoCl2, its constitutive expression is essential for CoCl2-induced PCD. The ATG5-mediated autophagic PCD requires interplays with endoplasmic reticulum (ER) and/or mitochondria. In this process, ATG5 plays a central role in regulating ER stress protein CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP) and mitochondrial protein second mitochondria derived activator of caspases (Smac). Two pathways for autophagic PCD in cancer cells responding to hypoxia have been identified: ATG5/CHOP/Smac pathway and ATG5/Smac pathway, which are probably dependent on the context of cell lines. The former is more potent than the latter for the induction of PCD at the early stage of hypoxia, although the ultimate efficiency of both pathways is comparable. In addition, both pathways may require ATG5-mediated conversion of LC3-I into LC3-II. Therefore, we have defined two autophagy-mediated pathways for the PCD of cancer cells in hypoxia, which are dependent on ATG5, interplayed with ER and mitochondria and tightly regulated by hypoxic status. The findings provide a new evidence that autophagy may inhibit tumor cell proliferation through trigger of PCD, facilitating the development of novel anti-cancer drugs.
Keywords: Cobalt chloride; autophagy; endoplasmic reticulum stress; mitochondria; programmed cell death.
Figures

References
-
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. - PubMed
-
- Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. - PubMed
-
- Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
