The vascular plant-pathogenic bacterium Ralstonia solanacearum produces biofilms required for its virulence on the surfaces of tomato cells adjacent to intercellular spaces
- PMID: 26609568
- PMCID: PMC6638453
- DOI: 10.1111/mpp.12335
The vascular plant-pathogenic bacterium Ralstonia solanacearum produces biofilms required for its virulence on the surfaces of tomato cells adjacent to intercellular spaces
Abstract
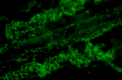
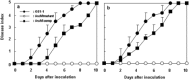
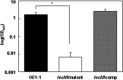
The mechanism of colonization of intercellular spaces by the soil-borne and vascular plant-pathogenic bacterium Ralstonia solanacearum strain OE1-1 after invasion into host plants remains unclear. To analyse the behaviour of OE1-1 cells in intercellular spaces, tomato leaves with the lower epidermis layers excised after infiltration with OE1-1 were observed under a scanning electron microscope. OE1-1 cells formed microcolonies on the surfaces of tomato cells adjacent to intercellular spaces, and then aggregated surrounded by an extracellular matrix, forming mature biofilm structures. Furthermore, OE1-1 cells produced mushroom-type biofilms when incubated in fluids of apoplasts including intercellular spaces, but not xylem fluids from tomato plants. This is the first report of biofilm formation by R. solanacearum on host plant cells after invasion into intercellular spaces and mushroom-type biofilms produced by R. solanacearum in vitro. Sugar application led to enhanced biofilm formation by OE1-1. Mutation of lecM encoding a lectin, RS-IIL, which reportedly exhibits affinity for these sugars, led to a significant decrease in biofilm formation. Colonization in intercellular spaces was significantly decreased in the lecM mutant, leading to a loss of virulence on tomato plants. Complementation of the lecM mutant with native lecM resulted in the recovery of mushroom-type biofilms and virulence on tomato plants. Together, our findings indicate that OE1-1 produces mature biofilms on the surfaces of tomato cells after invasion into intercellular spaces. RS-IIL may contribute to biofilm formation by OE1-1, which is required for OE1-1 virulence.
Keywords: Ralstonia solanacearum; biofilm; intercellular spaces; lectin; soil-borne vascular plant-pathogenic bacterium; virulence.
© 2015 BSPP AND JOHN WILEY & SONS LTD.
Figures






Similar articles
-
Ralfuranones contribute to mushroom-type biofilm formation by Ralstonia solanacearum strain OE1-1.Mol Plant Pathol. 2018 Apr;19(4):975-985. doi: 10.1111/mpp.12583. Epub 2017 Oct 10. Mol Plant Pathol. 2018. PMID: 28722830 Free PMC article.
-
Contribution of a lectin, LecM, to the quorum sensing signalling pathway of Ralstonia solanacearum strain OE1-1.Mol Plant Pathol. 2019 Mar;20(3):334-345. doi: 10.1111/mpp.12757. Epub 2018 Nov 6. Mol Plant Pathol. 2019. PMID: 30312504 Free PMC article.
-
The behavior of Ralstonia pseudosolanacearum strain OE1-1 and morphological changes of cells in tomato roots.J Plant Res. 2023 Jan;136(1):19-31. doi: 10.1007/s10265-022-01427-3. Epub 2022 Nov 25. J Plant Res. 2023. PMID: 36427093
-
Regulation Involved in Colonization of Intercellular Spaces of Host Plants in Ralstonia solanacearum.Front Plant Sci. 2017 Jun 8;8:967. doi: 10.3389/fpls.2017.00967. eCollection 2017. Front Plant Sci. 2017. PMID: 28642776 Free PMC article. Review.
-
How Ralstonia solanacearum Exploits and Thrives in the Flowing Plant Xylem Environment.Trends Microbiol. 2018 Nov;26(11):929-942. doi: 10.1016/j.tim.2018.06.002. Epub 2018 Jun 22. Trends Microbiol. 2018. PMID: 29941188 Review.
Cited by
-
Caffeic Acid in Tobacco Root Exudate Defends Tobacco Plants From Infection by Ralstonia solanacearum.Front Plant Sci. 2021 Aug 12;12:690586. doi: 10.3389/fpls.2021.690586. eCollection 2021. Front Plant Sci. 2021. PMID: 34456935 Free PMC article.
-
Dynamic expression of Ralstonia solanacearum virulence factors and metabolism-controlling genes during plant infection.BMC Genomics. 2021 Mar 9;22(1):170. doi: 10.1186/s12864-021-07457-w. BMC Genomics. 2021. PMID: 33750302 Free PMC article.
-
The transcription regulator ChpA affects the global transcriptome including quorum sensing-dependent genes in Ralstonia pseudosolanacearum strain OE1-1.Mol Plant Pathol. 2023 Nov;24(11):1370-1384. doi: 10.1111/mpp.13374. Epub 2023 Jul 14. Mol Plant Pathol. 2023. PMID: 37452484 Free PMC article.
-
A putative LysR-type transcriptional regulator PrhO positively regulates the type III secretion system and contributes to the virulence of Ralstonia solanacearum.Mol Plant Pathol. 2018 Jan 24;19(8):1808-19. doi: 10.1111/mpp.12660. Online ahead of print. Mol Plant Pathol. 2018. PMID: 29363870 Free PMC article.
-
Trehalose increases tomato drought tolerance, induces defenses, and increases resistance to bacterial wilt disease.PLoS One. 2022 Apr 27;17(4):e0266254. doi: 10.1371/journal.pone.0266254. eCollection 2022. PLoS One. 2022. PMID: 35476629 Free PMC article.
References
-
- Allen, C. , Huang, Y. and Sequeira, L. (1991) Cloning of genes affecting polygalacturonase production in Pseudomonas solanacearum . Mol. Plant–Microbe Interact. 4, 147–154.
-
- Araud‐Razou, I. , Vasse, J. , Montrozier, H. , Etchebar, C. and Trigalet, A. (1998) Detection and visualization of the major acidic exopolysaccharide of Ralstonia solanacearum and its role in tomato root infection and vascular colonization. Eur. J. Plant Pathol. 104, 795–809.
-
- Branda, S.S. , Vik, S. , Friedman, L. and Kolter, R. (2005) Biofilms: the matrix revisited. Trends Microbiol. 13, 20–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

