Assessment of cognitive safety in clinical drug development
- PMID: 26610416
- PMCID: PMC4863933
- DOI: 10.1016/j.drudis.2015.11.003
Assessment of cognitive safety in clinical drug development
Abstract
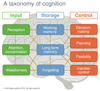
Cognitive impairment is increasingly recognised as an important potential adverse effect of medication. However, many drug development programmes do not incorporate sensitive cognitive measurements. Here, we review the rationale for cognitive safety assessment, and explain several basic methodological principles for measuring cognition during clinical drug development, including study design and statistical analysis, from Phase I through to postmarketing. The crucial issue of how cognition should be assessed is emphasized, especially the sensitivity of measurement. We also consider how best to interpret the magnitude of any identified effects, including comparison with benchmarks. We conclude by discussing strategies for the effective communication of cognitive risks.
Copyright © 2015 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Other than A.P.M. and J.P.R., all authors were employees of Cambridge Cognition Ltd at the time of writing. J.P.R. consults for Cambridge Cognition Ltd. P.J.N. is on the editorial board of Drug Discovery Today but was not involved in the review of this manuscript.
Figures

References
-
- Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105:S622–S627. - PubMed
-
- Fox C, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59:1477–1483. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

