Three-Dimensional Carotid Plaque MR Imaging
- PMID: 26610656
- PMCID: PMC4663679
- DOI: 10.1016/j.nic.2015.09.001
Three-Dimensional Carotid Plaque MR Imaging
Abstract
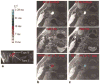
There has been significant progress made in 3-dimensional (3D) carotid plaque MR imaging techniques in recent years. Three-dimensional plaque imaging clearly represents the future in clinical use. With effective flow-suppression techniques, choices of different contrast weighting acquisitions, and time-efficient imaging approaches, 3D plaque imaging offers flexible imaging plane and view angle analysis, large coverage, multivascular beds capability, and even can be used in fast screening.
Keywords: 3D MR imaging; 3D MRA; 3D vessel wall imaging; Atherosclerosis imaging; Vessel wall imaging; Vulnerable plaque.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



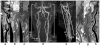
Similar articles
-
Improved vessel delineation in keyhole time-resolved contrast-enhanced MR angiography using a gadolinium doped flush.J Magn Reson Imaging. 2009 May;29(5):1147-53. doi: 10.1002/jmri.21761. J Magn Reson Imaging. 2009. PMID: 19388120 Clinical Trial.
-
Novel methodology for 3D reconstruction of carotid arteries and plaque characterization based upon magnetic resonance imaging carotid angiography data.Magn Reson Imaging. 2012 Oct;30(8):1068-82. doi: 10.1016/j.mri.2012.03.004. Epub 2012 May 21. Magn Reson Imaging. 2012. PMID: 22617149
-
Nonenhanced arterial spin labeled carotid MR angiography using three-dimensional radial balanced steady-state free precession imaging.J Magn Reson Imaging. 2015 Apr;41(4):1150-6. doi: 10.1002/jmri.24640. Epub 2014 Apr 16. J Magn Reson Imaging. 2015. PMID: 24737420
-
Low-Grade Carotid Stenosis: Implications of MR Imaging.Neuroimaging Clin N Am. 2016 Feb;26(1):129-45. doi: 10.1016/j.nic.2015.09.010. Epub 2015 Oct 23. Neuroimaging Clin N Am. 2016. PMID: 26610665 Review.
-
Analysis of Multicontrast Carotid Plaque MR Imaging.Neuroimaging Clin N Am. 2016 Feb;26(1):13-28. doi: 10.1016/j.nic.2015.09.002. Neuroimaging Clin N Am. 2016. PMID: 26610657 Review.
Cited by
-
Comparison of carotid atherosclerotic plaque characteristics between symptomatic patients with transient ischemic attack and stroke using high-resolution magnetic resonance imaging.BMC Cardiovasc Disord. 2022 Apr 21;22(1):190. doi: 10.1186/s12872-022-02624-7. BMC Cardiovasc Disord. 2022. PMID: 35448952 Free PMC article.
-
Interchangeable neck shape-specific coils for a clinically realizable anterior neck phased array system.Magn Reson Med. 2017 Dec;78(6):2460-2468. doi: 10.1002/mrm.26632. Epub 2017 Feb 10. Magn Reson Med. 2017. PMID: 28185303 Free PMC article.
-
High-resolution magnetic resonance vessel wall imaging provides new insights into Moyamoya disease.Front Neurosci. 2024 Apr 11;18:1375645. doi: 10.3389/fnins.2024.1375645. eCollection 2024. Front Neurosci. 2024. PMID: 38665292 Free PMC article. Review.
-
Segment-specific progression of carotid artery atherosclerosis: a magnetic resonance vessel wall imaging study.Neuroradiology. 2020 Feb;62(2):211-220. doi: 10.1007/s00234-019-02316-8. Epub 2019 Nov 13. Neuroradiology. 2020. PMID: 31720758
-
Fabrication of Customizable Intraplaque Hemorrhage Phantoms for Magnetic Resonance Imaging.Mol Imaging Biol. 2022 Oct;24(5):732-739. doi: 10.1007/s11307-022-01722-4. Epub 2022 Apr 29. Mol Imaging Biol. 2022. PMID: 35486294 Free PMC article.
References
-
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25(10):2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. - DOI - PubMed
-
- Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62(12):1081–91. doi: 10.1016/j.jacc.2013.06.015. - DOI - PubMed
-
- O'Leary DH, Polak JF. Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002;90(10C):18L–21L. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

