Genetic Variation in Toll-Interacting Protein Is Associated With Leprosy Susceptibility and Cutaneous Expression of Interleukin 1 Receptor Antagonist
- PMID: 26610735
- PMCID: PMC4779304
- DOI: 10.1093/infdis/jiv570
Genetic Variation in Toll-Interacting Protein Is Associated With Leprosy Susceptibility and Cutaneous Expression of Interleukin 1 Receptor Antagonist
Abstract
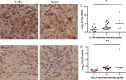
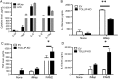
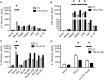
Leprosy is a chronic disease characterized by skin and peripheral nerve pathology and immune responses that fail to control Mycobacterium leprae. Toll-interacting protein (TOLLIP) regulates Toll-like receptor (TLR) and interleukin 1 receptor (IL-1R) signaling against mycobacteria. We analyzed messenger RNA (mRNA) expression of candidate immune genes in skin biopsy specimens from 85 individuals with leprosy. TOLLIP mRNA was highly and specifically correlated with IL-1R antagonist (IL-1Ra). In a case-control gene-association study with 477 cases and 1021 controls in Nepal, TOLLIP single-nucleotide polymorphism rs3793964 TT genotype was associated with increased susceptibility to leprosy (recessive, P = 1.4 × 10(-3)) and with increased skin expression of TOLLIP and IL-1Ra. Stimulation of TOLLIP-deficient monocytes with M. leprae produced significantly less IL-1Ra (P < .001), compared with control. These data suggest that M. leprae upregulates IL-1Ra by a TOLLIP-dependent mechanism. Inhibition of TOLLIP may decrease an individual's susceptibility to leprosy and offer a novel therapeutic target for IL-1-dependent diseases.
Keywords: IL-1; IL-1 receptor; IL-1Ra; Mycobacterium leprae; Skin immunity; TLR regulation; TOLLIP; genetics; immune evasion; leprosy.
© The Author 2015. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures


References
-
- Institute for Health Metrics and Evaluation. Global burden of leprosy. http://www.healthdata.org/search-gbd-data?s=leprosy Accessed 15 May 2015.
-
- World Health Organization. Global leprosy update, 2013; reducing disease burden. Wkly Epidemiol Rec 2014; 89:389–400. - PubMed
-
- Lahiri R, Randhawa B, Franken KL et al. Development of a mouse food pad model for detection of sub clinical leprosy. Lepr Rev 2011; 82:432–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

