Building from the Ground up: Basement Membranes in Drosophila Development
- PMID: 26610918
- PMCID: PMC4772872
- DOI: 10.1016/bs.ctm.2015.07.001
Building from the Ground up: Basement Membranes in Drosophila Development
Abstract
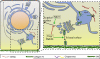
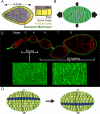
Basement membranes (BMs) are sheetlike extracellular matrices found at the basal surfaces of epithelial tissues. The structural and functional diversity of these matrices within the body endows them with the ability to affect multiple aspects of cell behavior and communication; for this reason, BMs are integral to many developmental processes. The power of Drosophila genetics, as applied to the BM, has yielded substantial insight into how these matrices influence development. Here, we explore three facets of BM biology to which Drosophila research has made particularly important contributions. First, we discuss how newly synthesized BM proteins are secreted to and assembled exclusively on basal epithelial surfaces. Next, we examine how regulation of the structural properties of the BM mechanically supports and guides tissue morphogenesis. Finally, we explore how BMs influence development through the modulation of several major signaling pathways.
Keywords: Basement membrane; Development; Drosophila; Morphogenesis; Secretion; Signaling.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



References
-
- Anitei M, Hoflack B. Exit from the trans-Golgi network: from molecules to mechanisms. Current Opinion in Cell Biology. 2011;23(4):443–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

