Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion
- PMID: 26611636
- PMCID: PMC4670995
- DOI: 10.1038/cr.2015.139
Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion
Abstract
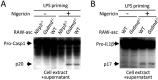
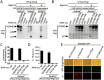
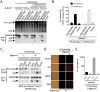
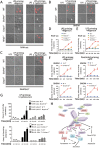
Inflammasome is an intracellular signaling complex of the innate immune system. Activation of inflammasomes promotes the secretion of interleukin 1β (IL-1β) and IL-18 and triggers pyroptosis. Caspase-1 and -11 (or -4/5 in human) in the canonical and non-canonical inflammasome pathways, respectively, are crucial for inflammasome-mediated inflammatory responses. Here we report that gasdermin D (GSDMD) is another crucial component of inflammasomes. We discovered the presence of GSDMD protein in nigericin-induced NLRP3 inflammasomes by a quantitative mass spectrometry-based analysis. Gene deletion of GSDMD demonstrated that GSDMD is required for pyroptosis and for the secretion but not proteolytic maturation of IL-1β in both canonical and non-canonical inflammasome responses. It was known that GSDMD is a substrate of caspase-1 and we showed its cleavage at the predicted site during inflammasome activation and that this cleavage was required for pyroptosis and IL-1β secretion. Expression of the N-terminal proteolytic fragment of GSDMD can trigger cell death and N-terminal modification such as tagging with Flag sequence disrupted the function of GSDMD. We also found that pro-caspase-1 is capable of processing GSDMD and ASC is not essential for GSDMD to function. Further analyses of LPS plus nigericin- or Salmonella typhimurium-treated macrophage cell lines and primary cells showed that apoptosis became apparent in Gsdmd(-/-) cells, indicating a suppression of apoptosis by pyroptosis. The induction of apoptosis required NLRP3 or other inflammasome receptors and ASC, and caspase-1 may partially contribute to the activation of apoptotic caspases in Gsdmd(-/-) cells. These data provide new insights into the molecular mechanisms of pyroptosis and reveal an unexpected interplay between apoptosis and pyroptosis.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

