Characterizing the angiogenic activity of patients with single ventricle physiology and aortopulmonary collateral vessels
- PMID: 26611747
- PMCID: PMC4801745
- DOI: 10.1016/j.jtcvs.2015.10.001
Characterizing the angiogenic activity of patients with single ventricle physiology and aortopulmonary collateral vessels
Abstract
Objectives: Patients with single ventricle congenital heart disease often form aortopulmonary collateral vessels via an unclear mechanism. To gain insights into the pathogenesis of aortopulmonary collateral vessels, we correlated angiogenic factor levels with in vitro activity and angiographic aortopulmonary collateral assessment and examined whether patients with single ventricle physiology have increased angiogenic factors that can stimulate endothelial cell sprouting in vitro.
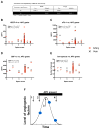
Methods: In patients with single ventricle physiology (n = 27) and biventricular acyanotic control patients (n = 21), hypoxia-inducible angiogenic factor levels were measured in femoral venous and arterial plasma at cardiac catheterization. To assess plasma angiogenic activity, we used a 3-dimensional in vitro cell sprouting assay that recapitulates angiogenic sprouting. Aortopulmonary collateral angiograms were graded using a 4-point scale.
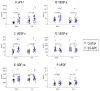
Results: Compared with controls, patients with single ventricle physiology had increased vascular endothelial growth factor (artery: 58.7 ± 1.2 pg/mL vs 35.3 ± 1.1 pg/mL, P < .01; vein: 34.8 ± 1.1 pg/mL vs 21 ± 1.2 pg/mL, P < .03), stromal-derived factor 1-alpha (artery: 1901.6 ± 1.1 pg/mL vs 1542.6 ± 1.1 pg/mL, P < .03; vein: 2092.8 pg/mL ± 1.1 vs 1752.9 ± 1.1 pg/mL, P < .02), and increased arterial soluble fms-like tyrosine kinase-1, a regulatory vascular endothelial growth factor receptor (612.3 ± 1.2 pg/mL vs 243.1 ± 1.2 pg/mL, P < .003). Plasma factors and sprout formation correlated poorly with aortopulmonary collateral severity.
Conclusions: We are the first to correlate plasma angiogenic factor levels with angiography and in vitro angiogenic activity in patients with single ventricle disease with aortopulmonary collaterals. Patients with single ventricle disease have increased stromal-derived factor 1-alpha and soluble fms-like tyrosine kinase-1, and their roles in aortopulmonary collateral formation require further investigation. Plasma factors and angiogenic activity correlate poorly with aortopulmonary collateral severity in patients with single ventricles, suggesting complex mechanisms of angiogenesis.
Keywords: aortopulmonary collateral; congenital heart disease; single ventricle.
Published by Elsevier Inc.
Conflict of interest statement
No potential conflicts of interest exist
None of the authors has any financial or other conflicts of interests.
Figures


Comment in
-
Aortopulmonary collaterals: Angiogenesis, or a whole lot more?J Thorac Cardiovasc Surg. 2016 Apr;151(4):1135-6. doi: 10.1016/j.jtcvs.2015.10.086. Epub 2015 Oct 28. J Thorac Cardiovasc Surg. 2016. PMID: 26621315 No abstract available.
References
-
- Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation. 2007 Feb 13;115(6):800–12. cited 4/17/2015 11:08:17 AM; 4/17/2015 11:08:17 AM. - PubMed
-
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002 Jun 19;39(12):1890–900. - PubMed
-
- Bradley SM, McCall MM, Sistino JJ, Radtke WA. Aortopulmonary collateral flow in the fontan patient: Does it matter? Ann Thorac Surg. 2001 Aug;72(2):408–15. - PubMed
-
- Kanter KR, Vincent RN. Management of aortopulmonary collateral arteries in fontan patients: Occlusion improves clinical outcome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2002;5:48–54. - PubMed
-
- McElhinney DB, Reddy VM, Tworetzky W, Petrossian E, Hanley FL, Moore P. Incidence and implications of systemic to pulmonary collaterals after bidirectional cavopulmonary anastomosis. Ann Thorac Surg. 2000 Apr;69(4):1222–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

