Estimation of passive and active properties in the human heart using 3D tagged MRI
- PMID: 26611908
- PMCID: PMC5021775
- DOI: 10.1007/s10237-015-0748-z
Estimation of passive and active properties in the human heart using 3D tagged MRI
Abstract
Advances in medical imaging and image processing are paving the way for personalised cardiac biomechanical modelling. Models provide the capacity to relate kinematics to dynamics and-through patient-specific modelling-derived material parameters to underlying cardiac muscle pathologies. However, for clinical utility to be achieved, model-based analyses mandate robust model selection and parameterisation. In this paper, we introduce a patient-specific biomechanical model for the left ventricle aiming to balance model fidelity with parameter identifiability. Using non-invasive data and common clinical surrogates, we illustrate unique identifiability of passive and active parameters over the full cardiac cycle. Identifiability and accuracy of the estimates in the presence of controlled noise are verified with a number of in silico datasets. Unique parametrisation is then obtained for three datasets acquired in vivo. The model predictions show good agreement with the data extracted from the images providing a pipeline for personalised biomechanical analysis.
Keywords: 3D tagged MRI; Cardiac mechanics; Parameter estimation; Patient-specific modelling.
Figures


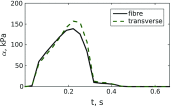
Similar articles
-
Analysis of passive cardiac constitutive laws for parameter estimation using 3D tagged MRI.Biomech Model Mechanobiol. 2015 Aug;14(4):807-28. doi: 10.1007/s10237-014-0638-9. Epub 2014 Dec 16. Biomech Model Mechanobiol. 2015. PMID: 25510227 Free PMC article.
-
A monolithic 3D-0D coupled closed-loop model of the heart and the vascular system: Experiment-based parameter estimation for patient-specific cardiac mechanics.Int J Numer Method Biomed Eng. 2017 Aug;33(8):e2842. doi: 10.1002/cnm.2842. Epub 2017 Feb 16. Int J Numer Method Biomed Eng. 2017. PMID: 27743468
-
Investigating the reference domain influence in personalised models of cardiac mechanics : Effect of unloaded geometry on cardiac biomechanics.Biomech Model Mechanobiol. 2021 Aug;20(4):1579-1597. doi: 10.1007/s10237-021-01464-2. Epub 2021 May 28. Biomech Model Mechanobiol. 2021. PMID: 34047891
-
Assessment of myocardial systolic function by tagged magnetic resonance imaging.J Cardiovasc Magn Reson. 2000;2(1):57-66. doi: 10.3109/10976640009148674. J Cardiovasc Magn Reson. 2000. PMID: 11545108 Review.
-
Assessment of myocardial function: a review of quantification methods and results using tagged MRI.J Cardiovasc Magn Reson. 2005;7(2):501-16. doi: 10.1081/jcmr-200053610. J Cardiovasc Magn Reson. 2005. PMID: 15881535 Review.
Cited by
-
Estimating prognosis in patients with acute myocardial infarction using personalized computational heart models.Sci Rep. 2017 Oct 19;7(1):13527. doi: 10.1038/s41598-017-13635-2. Sci Rep. 2017. PMID: 29051544 Free PMC article.
-
A Contemporary Look at Biomechanical Models of Myocardium.Annu Rev Biomed Eng. 2019 Jun 4;21:417-442. doi: 10.1146/annurev-bioeng-062117-121129. Annu Rev Biomed Eng. 2019. PMID: 31167105 Free PMC article. Review.
-
Using physiologically based models for clinical translation: predictive modelling, data interpretation or something in-between?J Physiol. 2016 Dec 1;594(23):6849-6863. doi: 10.1113/JP272003. Epub 2016 Jul 3. J Physiol. 2016. PMID: 27121495 Free PMC article. Review.
-
Bayesian optimisation for efficient parameter inference in a cardiac mechanics model of the left ventricle.Int J Numer Method Biomed Eng. 2022 May;38(5):e3593. doi: 10.1002/cnm.3593. Epub 2022 Apr 7. Int J Numer Method Biomed Eng. 2022. PMID: 35302293 Free PMC article.
-
An Implementation of Patient-Specific Biventricular Mechanics Simulations With a Deep Learning and Computational Pipeline.Front Physiol. 2021 Sep 16;12:716597. doi: 10.3389/fphys.2021.716597. eCollection 2021. Front Physiol. 2021. PMID: 34603077 Free PMC article.
References
-
- Asner L, Hadjicharalambous M, Lee J, Nordsletten D (2015) STACOM Challenge: simulating left ventricular mechanics in the canine heart. In: Statistical atlases and computational models of the heart-imaging and modelling challenges, vol 8896. Springer, pp 123–134
-
- Babuska I. The finite element method with Lagrangian multipliers. Numer Math. 1973;20(3):179–192. doi: 10.1007/BF01436561. - DOI
-
- Bonet J, Wood R. Nonlinear continuum mechanics for finite element analysis. Cambridge: Cambridge University Press; 2008.
-
- Brenner SC, Scott R. The mathematical theory of finite element methods. Berlin: Springer; 2008.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

