Enkephalin analogues with N-phenyl-N-(piperidin-2-ylmethyl)propionamide derivatives: Synthesis and biological evaluations
- PMID: 26611918
- PMCID: PMC4873255
- DOI: 10.1016/j.bmcl.2015.10.081
Enkephalin analogues with N-phenyl-N-(piperidin-2-ylmethyl)propionamide derivatives: Synthesis and biological evaluations
Abstract
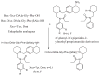
N-Phenyl-N-(piperidin-2-ylmethyl)propionamide based bivalent ligands are unexplored for the design of opioid based ligands. Two series of hybrid molecules bearing N-phenyl-N-(piperidin-2-ylmethyl)propionamide derived small molecules conjugated with an enkephalin analogues with and without a linker (β-alanine) were designed and synthesized. Both bivalent ligand series exhibited remarkable binding affinities from nanomolar to subnanomolar range at both μ and δ opioid receptors and displayed potent agonist activities as well. The replacement of Tyr with Dmt and introduction of a linker between the small molecule and enkephalin analogue resulted in highly potent ligands. Both series of ligands showed excellent binding affinities at both μ (0.6-0.9nM) and δ (0.2-1.2nM) opioid receptors respectively. Similarly, these bivalent ligands exhibited potent agonist activities in both MVD and GPI assays. Ligand 17 was evaluated for in vivo antinociceptive activity in non-injured rats following spinal administration. Ligand 17 was not significantly effective in alleviating acute pain. The most likely explanations for this low intrinsic efficacy in vivo despite high in vitro binding affinity, moderate in vitro activity are (i) low potency suggesting that higher doses are needed; (ii) differences in experimental design (i.e. non-neuronal, high receptor density for in vitro preparations versus CNS site of action in vitro); (iii) pharmacodynamics (i.e. engaging signalling pathways); (iv) pharmacokinetics (i.e. metabolic stability). In summary, our data suggest that further optimisation of this compound 17 is required to enhance intrinsic antinociceptive efficacy.
Keywords: Enkephalin; Opioid receptors; Opioids; Pain.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
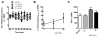

Similar articles
-
Design and synthesis of novel bivalent ligands (MOR and DOR) by conjugation of enkephalin analogues with 4-anilidopiperidine derivatives.Bioorg Med Chem Lett. 2015 Oct 15;25(20):4683-8. doi: 10.1016/j.bmcl.2015.07.064. Epub 2015 Jul 29. Bioorg Med Chem Lett. 2015. PMID: 26323872 Free PMC article.
-
Development of novel enkephalin analogues that have enhanced opioid activities at both mu and delta opioid receptors.J Med Chem. 2007 Nov 1;50(22):5528-32. doi: 10.1021/jm061465o. Epub 2007 Oct 10. J Med Chem. 2007. PMID: 17927164 Free PMC article.
-
Discovery of 5-substituted tetrahydronaphthalen-2yl-methyl with N-phenyl-N-(piperidin-4-yl)propionamide derivatives as potent opioid receptor ligands.Bioorg Med Chem. 2015 Sep 15;23(18):6185-94. doi: 10.1016/j.bmc.2015.07.071. Epub 2015 Aug 4. Bioorg Med Chem. 2015. PMID: 26299827 Free PMC article.
-
Design synthesis and structure-activity relationship of 5-substituted (tetrahydronaphthalen-2yl)methyl with N-phenyl-N-(piperidin-2-yl)propionamide derivatives as opioid ligands.Bioorg Med Chem. 2016 Jan 15;24(2):85-91. doi: 10.1016/j.bmc.2015.11.030. Epub 2015 Nov 23. Bioorg Med Chem. 2016. PMID: 26712115 Free PMC article.
-
Biphalin-A Potent Opioid Agonist-As a Panacea for Opioid System-Dependent Pathophysiological Diseases?Int J Mol Sci. 2021 Oct 21;22(21):11347. doi: 10.3390/ijms222111347. Int J Mol Sci. 2021. PMID: 34768778 Free PMC article. Review.
Cited by
-
Ultrasonic-induced synthesis of novel diverse arylidenes via Knoevenagel condensation reaction. Antitumor, QSAR, docking and DFT assessment.RSC Adv. 2023 Oct 10;13(42):29749-29767. doi: 10.1039/d3ra05799b. eCollection 2023 Oct 4. RSC Adv. 2023. PMID: 37822658 Free PMC article.
-
Synthesis and Opioid Activity of Tyr1 -ψ[(Z)CF=CH]-Gly2 and Tyr1 -ψ[(S)/(R)-CF3 CH-NH]-Gly2 Leu-enkephalin Fluorinated Peptidomimetics.ChemMedChem. 2017 Apr 20;12(8):571-576. doi: 10.1002/cmdc.201700103. Epub 2017 Apr 5. ChemMedChem. 2017. PMID: 28296145 Free PMC article.
-
Tyr1-ψ[( Z)CF═CH]-Gly2 Fluorinated Peptidomimetic Improves Distribution and Metabolism Properties of Leu-Enkephalin.ACS Chem Neurosci. 2018 Jul 18;9(7):1735-1742. doi: 10.1021/acschemneuro.8b00085. Epub 2018 Apr 19. ACS Chem Neurosci. 2018. PMID: 29648788 Free PMC article.
References
-
- Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TMD, Lemieux C, Chung NN, Coderre TJ. J Med Chem. 1999;42:3520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Research Materials

