Dexmedetomidine-midazolam versus Sufentanil-midazolam for Awake Fiberoptic Nasotracheal Intubation: A Randomized Double-blind Study
- PMID: 26612286
- PMCID: PMC4794886
- DOI: 10.4103/0366-6999.170260
Dexmedetomidine-midazolam versus Sufentanil-midazolam for Awake Fiberoptic Nasotracheal Intubation: A Randomized Double-blind Study
Abstract
Background: Awake fiberoptic intubation (AFOI) is usually performed in the management of the predicted difficult airway. The aim of this study was to evaluate the feasibility of dexmedetomidine with midazolam (DM) and sufentanil with midazolam (SM) for sedation for awake fiberoptic nasotracheal intubation.
Methods: Fifty patients with limited mouth opening scheduled for AFOI were randomly assigned to two groups (n = 25 per group) by a computer-generated randomization schedule. All subjects received midazolam 0.02 mg/kg as premedication and airway topical anesthesia with a modified "spray-as-you-go" technique. Group DM received dexmedetomidine at a loading dose of 0.5 μg/kg over 10 min followed by a continuous infusion of 0.25 μg·kg-1·h-1, whereas Group SM received sufentanil at a loading dose of 0.2 μg/kg over 10 min followed by a continuous infusion of 0.1 μg·kg-1·h-1. As necessary, since the end of the administration of the loading dose of the study drug, an additional dose of midazolam 0.5 mg at 2-min intervals was given to achieve a modified Observers' Assessment of Alertness/Sedation of 2-3. The quality of intubation conditions and adverse events were observed.
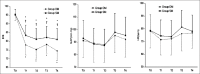
Results: The scores of ease of the AFOI procedure, patient's reaction during AFOI, coughing severity, tolerance after intubation, recall of the procedure and discomfort during the procedure were comparable in both groups (z = 0.572, 0.664, 1.297, 0.467, 0.895, and 0.188, respectively, P > 0.05). Hypoxic episodes similarly occurred in the two groups, but the first partial pressure of end-tidal CO2after intubation was higher in Group SM than that in Group DM (45.2 ± 4.2 mmHg vs. 42.2 ± 4.3 mmHg, t = 2.495, P < 0.05).
Conclusions: Both dexmedetomidine and sufentanil are effective as an adjuvant for AFOI under airway topical anesthesia combined with midazolam sedation, but respiratory depression is still a potential risk in the sufentanil regimen.
Figures
Comment in
-
Comparing Sedation Regimens for Awake Fiberoptic Intubation.Chin Med J (Engl). 2016 Feb 20;129(4):502-3. doi: 10.4103/0366-6999.176087. Chin Med J (Engl). 2016. PMID: 26879033 Free PMC article. No abstract available.
-
Authors' Reply to Letter to the Editor "Comparing Sedation Regimens for Awake Fiberoptic Intubation".Chin Med J (Engl). 2016 Feb 20;129(4):503-4. doi: 10.4103/0366-6999.176089. Chin Med J (Engl). 2016. PMID: 26879034 Free PMC article. No abstract available.
References
-
- Bailey PL, Streisand JB, East KA, East TD, Isern S, Hansen TW, et al. Differences in magnitude and duration of opioid-induced respiratory depression and analgesia with fentanyl and sufentanil. Anesth Analg. 1990;70:8–15. - PubMed
-
- Shen SL, Xie YH, Wang WY, Hu SF, Zhang YL. Comparison of dexmedetomidine and sufentanil for conscious sedation in patients undergoing awake fibreoptic nasotracheal intubation: A prospective, randomised and controlled clinical trial. Clin Respir J. 2014;8:100–7. - PubMed
-
- Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40. - PubMed
-
- Chu KS, Wang FY, Hsu HT, Lu IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol. 2010;27:36–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

