Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss
- PMID: 26612338
- PMCID: PMC4819614
- DOI: 10.1136/annrheumdis-2015-208093
Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss
Erratum in
-
Correction: Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss.Ann Rheum Dis. 2019 Jun;78(6):866. doi: 10.1136/annrheumdis-2015-208093corr1. Epub 2019 Mar 18. Ann Rheum Dis. 2019. PMID: 30885993 Free PMC article. No abstract available.
Abstract
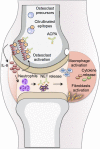
Objectives: Rheumatoid arthritis (RA)-specific anti-citrullinated protein/peptide antibodies (ACPAs) appear before disease onset and are associated with bone destruction. We aimed to dissect the role of ACPAs in osteoclast (OC) activation and to identify key cellular mediators in this process.
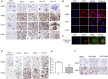
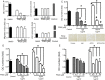
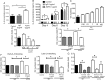
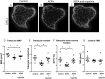
Methods: Polyclonal ACPA were isolated from the synovial fluid (SF) and peripheral blood of patients with RA. Monoclonal ACPAs were isolated from single SF B-cells of patients with RA. OCs were developed from blood cell precursors with or without ACPAs. We analysed expression of citrullinated targets and peptidylarginine deiminases (PAD) enzymes by immunohistochemistry and cell supernatants by cytometric bead array. The effect of an anti-interleukin (IL)-8 neutralising antibody and a pan-PAD inhibitor was tested in the OC cultures. Monoclonal ACPAs were injected into mice and bone structure was analysed by micro-CT before and after CXCR1/2 blocking with reparixin.
Results: Protein citrullination by PADs is essential for OC differentiation. Polyclonal ACPAs enhance OC differentiation through a PAD-dependent IL-8-mediated autocrine loop that is completely abolished by IL-8 neutralisation. Some, but not all, human monoclonal ACPAs derived from single SF B-cells of patients with RA and exhibiting distinct epitope specificities promote OC differentiation in cell cultures. Transfer of the monoclonal ACPAs into mice induced bone loss that was completely reversed by the IL-8 antagonist reparixin.
Conclusions: We provide novel insights into the key role of citrullination and PAD enzymes during OC differentiation and ACPA-induced OC activation. Our findings suggest that IL8-dependent OC activation may constitute an early event in the initiation of the joint specific inflammation in ACPA-positive RA.
Keywords: Ant-CCP; Autoantibodies; Early Rheumatoid Arthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Comment in
-
Rheumatoid arthritis: Osteoclasts and ACPAs--the joint link.Nat Rev Rheumatol. 2016 Feb;12(2):69. doi: 10.1038/nrrheum.2015.177. Epub 2015 Dec 17. Nat Rev Rheumatol. 2016. PMID: 26676084 No abstract available.
-
Bone loss, pain and inflammation: three faces of ACPA in RA pathogenesis.Ann Rheum Dis. 2016 Apr;75(4):637-9. doi: 10.1136/annrheumdis-2015-208308. Epub 2016 Jan 14. Ann Rheum Dis. 2016. PMID: 26768407 No abstract available.
-
Pathogenic effector functions of ACPA: Where do we stand?Ann Rheum Dis. 2019 Jun;78(6):716-721. doi: 10.1136/annrheumdis-2019-215337. Epub 2019 Apr 20. Ann Rheum Dis. 2019. PMID: 31005898 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

