iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium
- PMID: 26612359
- PMCID: PMC4727567
- DOI: 10.1111/jcmm.12725
iPSC-derived human cardiac progenitor cells improve ventricular remodelling via angiogenesis and interstitial networking of infarcted myocardium
Abstract
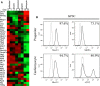
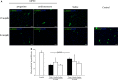
We investigate the effects of myocardial transplantation of human induced pluripotent stem cell (iPSC)-derived progenitors and cardiomyocytes into acutely infarcted myocardium in severe combined immune deficiency mice. A total of 2 × 10(5) progenitors, cardiomyocytes or cell-free saline were injected into peri-infarcted anterior free wall. Sham-operated animals received no injection. Myocardial function was assessed at 2-week and 4-week post-infarction by using echocardiography and pressure-volume catheterization. Early myocardial remodelling was observed at 2-week with echocardiography derived stroke volume (SV) in saline (20.45 ± 7.36 μl, P < 0.05) and cardiomyocyte (19.52 ± 3.97 μl, P < 0.05) groups, but not in progenitor group (25.65 ± 3.61 μl), significantly deteriorated as compared to sham control group (28.41 ± 4.41 μl). Consistently, pressure-volume haemodynamic measurements showed worsening chamber dilation in saline (EDV: 23.24 ± 5.01 μl, P < 0.05; ESV: 17.08 ± 5.82 μl, P < 0.05) and cardiomyocyte (EDV: 26.45 ± 5.69 μl, P < 0.05; ESV: 18.03 ± 6.58 μl, P < 0.05) groups by 4-week post-infarction as compared to control (EDV: 15.26 ± 2.96 μl; ESV: 8.41 ± 2.94 μl). In contrast, cardiac progenitors (EDV: 20.09 ± 7.76 μl; ESV: 13.98 ± 6.74 μl) persistently protected chamber geometry against negative cardiac remodelling. Similarly, as compared to sham control (54.64 ± 11.37%), LV ejection fraction was preserved in progenitor group from 2-(38.68 ± 7.34%) to 4-week (39.56 ± 13.26%) while cardiomyocyte (36.52 ± 11.39%, P < 0.05) and saline (35.34 ± 11.86%, P < 0.05) groups deteriorated early at 2-week. Improvements of myocardial function in the progenitor group corresponded to increased vascularization (16.12 ± 1.49/mm(2) to 25.48 ± 2.08/mm(2) myocardial tissue, P < 0.05) and coincided with augmented networking of cardiac telocytes in the interstitial space of infarcted zone.
Keywords: cardiac progenitors; cardiac repair; cardiomyocytes; interstitial cells; telocytes.
© 2015 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
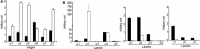



References
-
- Mehta A, Shim W. Cardiac stem cell therapy: stemness or commitment? Cell Transplant. 2013; 22: 1–14. - PubMed
-
- Shim W, Mehta A, Wong P, et al Critical path in cardiac stem cell therapy: an update on cell delivery. Cytotherapy. 2013; 15: 399–415. - PubMed
-
- Menasche P, Vanneaux V, Hagege A, et al Human embryonic stem cell‐derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015; 36: 2011–7. - PubMed
-
- Menasche P. Stem cell therapy for chronic heart failure: lessons from a 15‐year experience. C R Biol. 2011; 334: 489–96. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

