Rapid generation of sub-type, region-specific neurons and neural networks from human pluripotent stem cell-derived neurospheres
- PMID: 26613348
- PMCID: PMC4698048
- DOI: 10.1016/j.scr.2015.10.014
Rapid generation of sub-type, region-specific neurons and neural networks from human pluripotent stem cell-derived neurospheres
Abstract
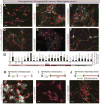
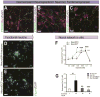
Stem cell-based neuronal differentiation has provided a unique opportunity for disease modeling and regenerative medicine. Neurospheres are the most commonly used neuroprogenitors for neuronal differentiation, but they often clump in culture, which has always represented a challenge for neurodifferentiation. In this study, we report a novel method and defined culture conditions for generating sub-type or region-specific neurons from human embryonic and induced pluripotent stem cells derived neurosphere without any genetic manipulation. Round and bright-edged neurospheres were generated in a supplemented knockout serum replacement medium (SKSRM) with 10% CO2, which doubled the expression of the NESTIN, PAX6 and FOXG1 genes compared with those cultured with 5% CO2. Furthermore, an additional step (AdSTEP) was introduced to fragment the neurospheres and facilitate the formation of a neuroepithelial-type monolayer that we termed the "neurosphederm". The large neural tube-type rosette (NTTR) structure formed from the neurosphederm, and the NTTR expressed higher levels of the PAX6, SOX2 and NESTIN genes compared with the neuroectoderm-derived neuroprogenitors. Different layers of cortical, pyramidal, GABAergic, glutamatergic, cholinergic neurons appeared within 27 days using the neurosphederm, which is a shorter period than in traditional neurodifferentiation-protocols (42-60 days). With additional supplements and timeline dopaminergic and Purkinje neurons were also generated in culture too. Furthermore, our in vivo results indicated that the fragmented neurospheres facilitated significantly better neurogenesis in severe combined immunodeficiency (SCID) mouse brains compared with the non-fragmented neurospheres. Therefore, this neurosphere-based neurodifferentiation protocol is a valuable tool for studies of neurodifferentiation, neuronal transplantation and high throughput screening assays.
Keywords: Human embryonic stem cells; Induced pluripotent stem cells; Neuroectoderm; Neurogenesis; Neurons; Neurosphere.
Published by Elsevier B.V.
Figures




References
-
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. - PubMed
-
- Yagi T, Ito D, Okada Y, Akamatsu W, Nihei Y, Yoshizaki T, Yamanaka S, Okano H, Suzuki N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet. 2011;20(23):4530–4539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

