Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences
- PMID: 26613664
- PMCID: PMC4713283
- DOI: 10.1016/j.pt.2015.09.008
Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences
Abstract
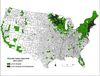
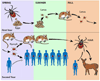
Ixodes ticks maintain a large and diverse array of human pathogens in the enzootic cycle, including Borrelia burgdorferi and Babesia microti. Despite the poor ecological fitness of B. microti, babesiosis has recently emerged in areas endemic for Lyme disease. Studies in ticks, reservoir hosts, and humans indicate that coinfection with B. burgdorferi and B. microti is common, promotes transmission and emergence of B. microti in the enzootic cycle, and causes greater disease severity and duration in humans. These interdisciplinary studies may serve as a paradigm for the study of other vector-borne coinfections. Identifying ecological drivers of pathogen emergence and host factors that fuel disease severity in coinfected individuals will help guide the design of effective preventative and therapeutic strategies.
Keywords: Babesia; Borrelia; Lyme disease; babesiosis; coinfection; tick.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Rynkiewicz EC, et al. An ecosystem approach to understanding and managing within-host parasite community dynamics. Trends Parasitol. 2015;31:212–221. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

