The wavy Mutation Maps to the Inositol 1,4,5-Trisphosphate 3-Kinase 2 (IP3K2) Gene of Drosophila and Interacts with IP3R to Affect Wing Development
- PMID: 26613949
- PMCID: PMC4751550
- DOI: 10.1534/g3.115.024307
The wavy Mutation Maps to the Inositol 1,4,5-Trisphosphate 3-Kinase 2 (IP3K2) Gene of Drosophila and Interacts with IP3R to Affect Wing Development
Abstract
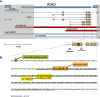
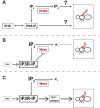
Inositol 1,4,5-trisphosphate (IP3) regulates a host of biological processes from egg activation to cell death. When IP3-specific receptors (IP3Rs) bind to IP3, they release calcium from the ER into the cytoplasm, triggering a variety of cell type- and developmental stage-specific responses. Alternatively, inositol polyphosphate kinases can phosphorylate IP3; this limits IP3R activation by reducing IP3 levels, and also generates new signaling molecules altogether. These divergent pathways draw from the same IP3 pool yet cause very different cellular responses. Therefore, controlling the relative rates of IP3R activation vs. phosphorylation of IP3 is essential for proper cell functioning. Establishing a model system that sensitively reports the net output of IP3 signaling is crucial for identifying the controlling genes. Here we report that mutant alleles of wavy (wy), a classic locus of the fruit fly Drosophila melanogaster, map to IP3 3-kinase 2 (IP3K2), a member of the inositol polyphosphate kinase gene family. Mutations in wy disrupt wing structure in a highly specific pattern. RNAi experiments using GAL4 and GAL80(ts) indicated that IP3K2 function is required in the wing discs of early pupae for normal wing development. Gradations in the severity of the wy phenotype provide high-resolution readouts of IP3K2 function and of overall IP3 signaling, giving this system strong potential as a model for further study of the IP3 signaling network. In proof of concept, a dominant modifier screen revealed that mutations in IP3R strongly suppress the wy phenotype, suggesting that the wy phenotype results from reduced IP4 levels, and/or excessive IP3R signaling.
Keywords: Cam; GAL80; IP3K1; Ipk2; genetic interaction.
Copyright © 2016 Dean et al.
Figures



References
-
- Azpiazu N., Morata G., 2000. Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development 127: 2685–2693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
