Adeno-associated viral vectors for the treatment of hemophilia
- PMID: 26614390
- PMCID: PMC4802375
- DOI: 10.1093/hmg/ddv475
Adeno-associated viral vectors for the treatment of hemophilia
Abstract
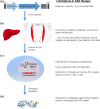
Gene transfer studies for the treatment of hemophilia began more than two decades ago. A large body of pre-clinical work evaluated a variety of vectors and target tissues, but by the start of the new millennium it became evident that adeno-associated viral (AAV)-mediated gene transfer to the liver held great promise as a therapeutic tool. The transition to the clinical arena uncovered a number of unforeseen challenges, mainly in the form of a human-specific immune response against the vector that poses a significant limitation in the application of this technology. While the full nature of this response has not been elucidated, long-term expression of therapeutic levels of factor IX is already a reality for a small number of patients. Extending this success to a greater number of hemophilia B patients remains a major goal of the field, as well as translating this strategy to clinical therapy for hemophilia A. This review summarizes the progress of AAV-mediated gene therapy for the hemophilias, along with its upcoming prospects and challenges.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Soucie J.M., Nuss R., Evatt B., Abdelhak A., Cowan L., Hill H., Kolakoski M., Wilber N. (2000) Mortality among males with hemophilia: relations with source of medical care. The Hemophilia Surveillance System Project Investigators. Blood, 96, 437–442. - PubMed
-
- Aggeler P.M., White S.G., Glendening M.B., Page E.W., Leake T.B., Bates G. (1952) Plasma thromboplastin component (PTC) deficiency; a new disease resembling hemophilia. Proc. Soc. Exp. Biol. Med., 79, 692–694. - PubMed
-
- Soucie J.M., Evatt B., Jackson D. (1998) Occurrence of hemophilia in the United States. The Hemophilia Surveillance System Project Investigators. Am. J. Hematol., 59, 288–294. - PubMed
-
- Gitschier J., Wood W.I., Goralka T.M., Wion K.L., Chen E.Y., Eaton D.H., Vehar G.A., Capon D.J., Lawn R.M. (1984) Characterization of the human factor VIII gene. Nature, 312, 326–330. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

